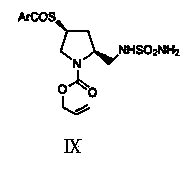
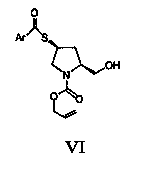
Chiral pyrrolidine compound and preparation method thereof
A technology of compound and pyrrolidine, which is applied in the field of chiral pyrrolidine compound and its preparation, can solve the problems affecting the manufacturing cost of doripenem chiral side chains, the difficulty of high-content side chain compounds, and the difficulty of industrialization, etc., to achieve Easy to purify, reduce dosage, good effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Under nitrogen atmosphere, mix compound I (131.1g, 1mol) with methanol (655ml) to obtain a suspension, stir for 5 minutes and mix well, then cool to -10°C, control the temperature at -5~0°C and add thionyl chloride (119g) dropwise , 1mol), add it in 30 minutes. Stir at 15~20°C for 5 hours. After the reaction is detected by TLC, the temperature is reduced to -5°C, and the temperature is controlled below 10°C by adding sodium hydroxide to adjust the pH to 7, adding potassium carbonate (165.9g, 1.2mol), and continuing to control the temperature for 5~10 Add allyl chloroformate (120.5g, 1mol) dropwise at ℃ and stir for 2.5 hours at the same temperature. After the reaction is detected by TLC, remove potassium chloride and excess potassium carbonate by filtration, add water (700ml) and dichloromethane 390ml to the filtrate and stir well . The organic layer was separated, and the aqueous layer was extracted once with 200 ml of dichloromethane. The organic layer was washed with...
Embodiment 2
[0072] Under nitrogen atmosphere, mix compound I (131.1g, 1mol) with methanol (655ml) to obtain a suspension, stir for 5 minutes and mix well, control the temperature at 15~20℃ and add thionyl chloride (238g, 2mol) dropwise for 50 minutes Finish adding. Stir at 35~40°C for 2 hours. After the reaction is detected by TLC, the temperature is reduced to 0°C, and the temperature is controlled below 10°C by adding potassium hydroxide to adjust the pH to 8, adding triethylamine (151.8g, 1.5mol), and continuing to control the temperature for 20-25 Allyl chloroformate (156.7 g, 1.3 mol) was added dropwise at °C, and stirred at the same temperature for 0.5 hours. After the reaction was detected by TLC, 199.5 g of III was obtained as in Example 1, with a yield of 87.0%.
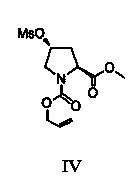
[0073] (B) Compound III → Compound IV
Embodiment 3
[0075] Under a nitrogen atmosphere, mix compound III (200g, 0.87mol) obtained in Example 1 with dichloromethane (1000ml), then add triethylamine (132g, 1.30mol), control the temperature at -20~-10℃, and add dropwise Methanesulfonyl chloride (148.4 g, 1.3 mol) was dripped and the mixture was stirred for 30 minutes. After the reaction was detected by TLC, the reaction mixture was continuously washed with 300 ml of 2N hydrochloric acid and saturated brine, dried over magnesium sulfate, and concentrated in vacuo to obtain an oily product, that is, compound IV was 255.1 g, and the yield was 95.4%.
[0076] 1 H NMR (500MHz, CDCl 3 )Δ: 5.64(m,1H), 5.34–5.20 (d, 2H), 4.89(m,1H), 4.21(m,1H), 3.88 (s,3 H), 3.85 (m,1 H), 3.82 -3.67 (m, 3 H), 3.12 (s, 3H), 2.10-2.09 (m, 2 H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap