A method of producing phosphoric acid and clean gypsum
A technology of gypsum and phosphoric acid, applied in chemical instruments and methods, phosphorus compounds, calcium/strontium/barium sulfate, etc., can solve problems such as unreasonable heat utilization, loss, instability, etc., and achieve good filtration performance and good crystallization conditions , the effect of reducing losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
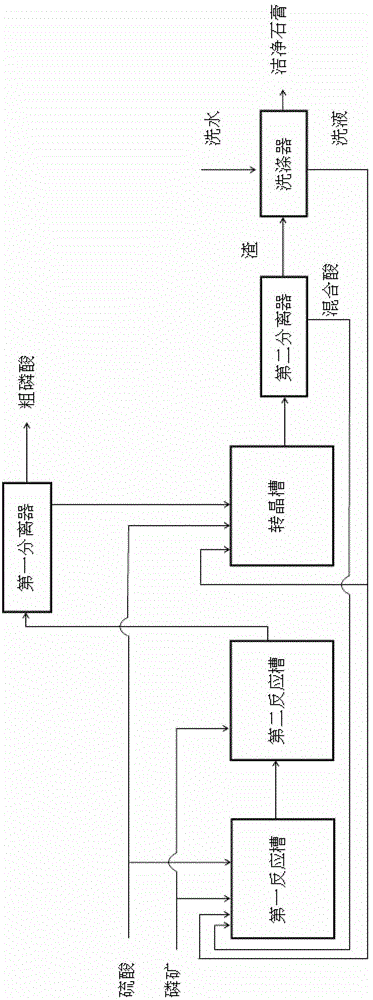
[0031] In the present embodiment, containing P in the phosphate rock 2 o 5 28.6%, CaO 39%, and MgO 2% (mass percentage), the particle size of phosphate rock meets 80% of the total phosphate rock feed through an 80-mesh sieve; the concentration of sulfuric acid is 80 wt%, and the total feed amount (mass) of sulfuric acid is 99.3% of the theoretical consumption (mass) of sulfuric acid by CaO and MgO in the total amount of phosphate rock. The process steps are as follows:
[0032] (1) Phosphate rock acid hydrolysis process
[0033] 85% of the total feed amount (mass) of powder phosphate rock, 40% of the total feed amount (mass) of sulfuric acid, and the P-containing powder returned by the second separator in step (5) 2 o 5 25.8 wt%, sulfuric acid 13 wt% mixed acid of phosphoric acid and sulfuric acid, returned from the scrubber containing P 2 o 5 20.2 wt%, sulfuric acid 10.1 wt% washing solution (in the initial stage of process start-up, the mixed acid of phosphoric acid...
Embodiment 2
[0044] In the present embodiment, phosphorus-containing ore in phosphate rock contains P 2 o 5 28.6%, CaO 39%, MgO 2% (mass percentage), the particle size of phosphate rock meets 80% of the total phosphate rock feed through the 80 mesh screen; the concentration of sulfuric acid is 98 wt%, the total feed amount of sulfuric acid (mass) It is 99.0% of the theoretical consumption (mass) of sulfuric acid by CaO and MgO in the total amount of phosphate rock. The process steps are as follows:
[0045] (1) Phosphate rock acid hydrolysis process
[0046] 80% of the total feed amount (mass) of powder phosphate rock, 35% of the total feed amount (mass) of sulfuric acid, and the P-containing 2 o 5 26 wt%, sulfuric acid 15.3 wt% mixed acid of phosphoric acid and sulfuric acid, return from the scrubber containing P 2 o 5 25.3 wt%, sulfuric acid 14.8 wt% washing solution (at the initial stage of process start-up, the mixed acid of phosphoric acid and sulfuric acid is 2 o 5 26 wt%...
Embodiment 3
[0057] In the present embodiment, containing P in the phosphate rock 2 o 5 33.6%, CaO 44.5%, MgO 0.5% (mass percentage), the particle size of phosphate rock meets 80% of the total amount of phosphate rock feeding through the 80 mesh screen; the concentration of sulfuric acid is 98 wt%, the total feeding amount of sulfuric acid (mass) It is 99.1% of the theoretical consumption (mass) of sulfuric acid by CaO and MgO in the total amount of powdered phosphate rock. The process steps are as follows:
[0058] (1) Phosphate rock acid hydrolysis process
[0059] 95% of the total feed amount (mass) of powder phosphate rock, 50% of the total feed amount (mass) of sulfuric acid, and the P-containing powder returned by the second separator in step (5) 2 o 5 31.8 wt%, sulfuric acid 10.7wt% mixed acid of phosphoric acid and sulfuric acid, return from the scrubber containing P 2 o 5 24.8 wt%, sulfuric acid 8.3 wt% washing solution (in the initial stage of process start-up, the mixed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com