Heterocyclic compound as well as preparation method and application thereof
A technology for heterocyclic compounds and compounds, applied in the field of compounds, can solve the problems of influence, low yield, long route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]
[0061] (1) Suspend the compound of formula 2a (1.0mmol) in the solvent THF (tetrahydrofuran) (5mL), add TMSCl (trimethylchlorosilane) (1.1mmol) at room temperature, then cool down to -60°C, and dropwise add excess n-Butyllithium (2.5M hexane solution, 3.2mmol), kept stirring at -60°C for 2h after dropping; while preparing the compound of formula 2b, the H on the 7-position of the compound of formula 2b was removed;
[0062] (2) Prepare another reaction bottle, add electrophile ethylene oxide (2.0mmol) and THF (10mL), and stir at room temperature to obtain an electrophile solution;
[0063] (3) Pre-cool the electrophile solution prepared in step (2) to -60°C and add it to step (1), continue to stir at -60°C for 3h; then gradually heat to -20°C, add acetic acid (3mmol) quenching reaction,
[0064] (4) The reaction solution was washed successively with water, saturated aqueous sodium bicarbonate solution, and saturated brine. Finally, the obtained organic phase is c...
Embodiment 2
[0066]
[0067] (1) Suspend the compound of formula 2a (1.0mmol) in THF (5mL), add TMSCl (1.1mmol) at room temperature, then cool down to 0°C, add NaH (60% suspended in mineral oil, 1.1mmol), then Stir at 0°C for 30 min to obtain the compound of formula 2b;
[0068] (2) Cool down to -60°C, add n-butyllithium (2.5M hexane solution, 2.2mmol) dropwise, and stir at -60°C for 2 hours after dropping, to remove the H on the 7-position of the compound of formula 2b.
[0069] (3) Prepare another reaction bottle, add ethylene oxide (2.0mmol) and THF (10mL), and stir to obtain an electrophilic reagent solution;
[0070] (4) Precool the electrophile solution obtained by stirring evenly to -60° C., and add it to the lithiated mixture in step (2). The mixture was continued to be stirred at -60°C for 3 h, gradually heated to -20°C, acetic acid (3 mmol) was added to quench the reaction, and the reaction solution was washed successively with water, saturated aqueous sodium bicarbonate solu...
Embodiment 3
[0072]
[0073] Suspend the compound of formula 2a (1.0mmol) in THF (5mL), add TMSCl (1.1mmol) at room temperature, then cool down to 0°C, add N,N-diisopropylethylamine (2.0mmol), and then Stir at ℃ for 30 min, then cool down to -60°C, add n-butyllithium (2.5M hexane solution, 2.2mmol) dropwise, and stir at -60°C for 2h after dropping.
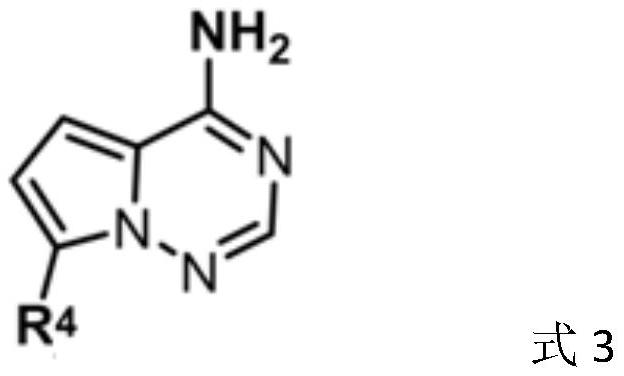
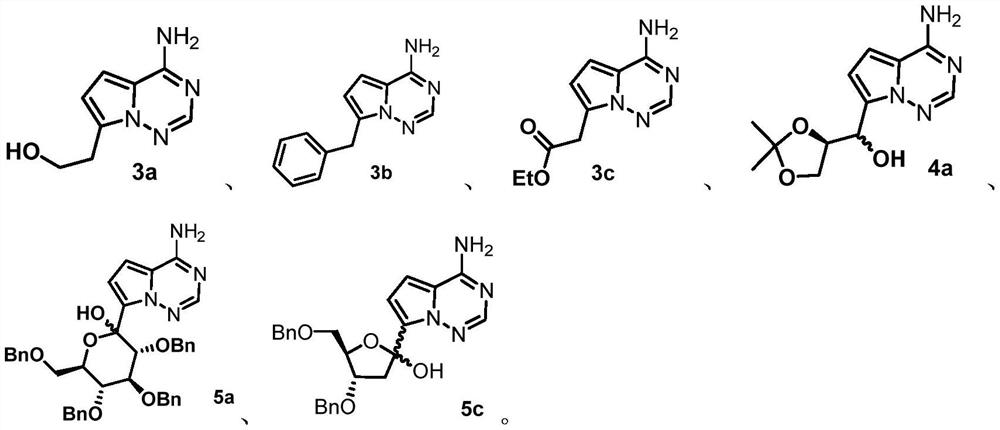
[0074] Prepare another reaction vial, add ethylene oxide (2.0 mmol) and THF (10 mL), and pre-cool the solution to -60°C, add to the above lithiated mixture. The mixture was continued to be stirred at -60°C for 3 h, gradually heated to -20°C, acetic acid (3 mmol) was added to quench the reaction, and the reaction solution was washed successively with water, saturated aqueous sodium bicarbonate solution, and saturated brine. Finally, the obtained organic phase was concentrated and then column chromatographed to obtain the compound of formula 3a with a yield of 71%, and the analytical data was the same as that of the standard sample of compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com