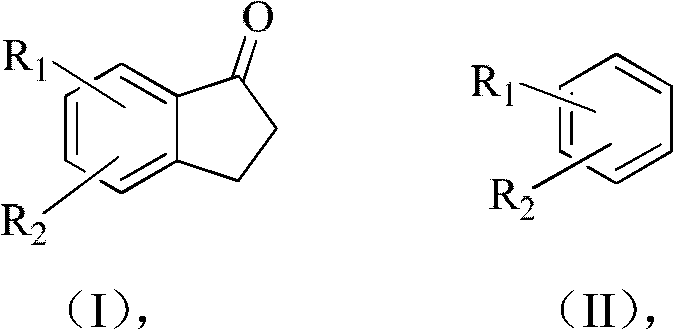
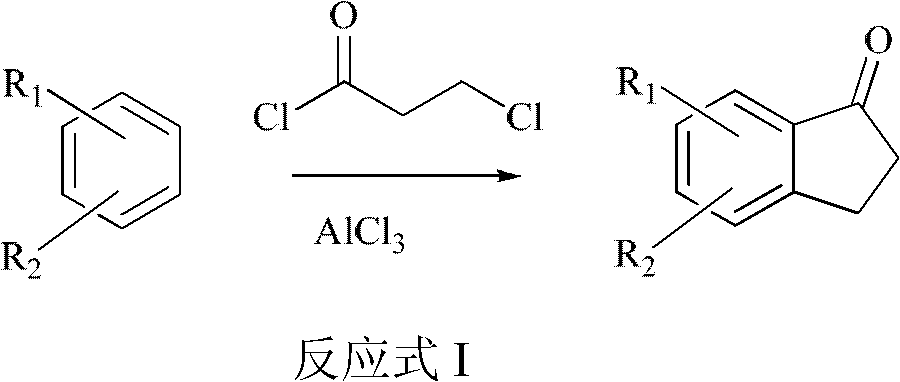
A method for preparing 2,3-dihydro-1-indanone and derivatives thereof
A technology of indanone and dihydrogen, applied in the field of preparation 2, can solve the problems of easy blockage of reactor pipes, large quantities, and easy fires, and achieve the effects of reducing sublimation, reducing pollution, and low equipment occupancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
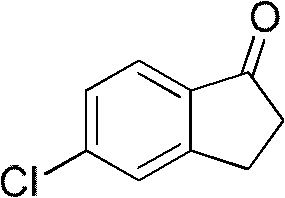
[0028] The preparation of embodiment 1.5-chloro-2,3-dihydro-1-indanone
[0029]
[0030] Add chlorobenzene (200g, 1.78mol), aluminum chloride (670g, 5mol), NaCl (110g, 1.88mol) in a 1-liter three-necked flask, stir, and dropwise add 3-chloropropionyl chloride ( 237g, 1.87mol), the mol ratio of chlorobenzene and 3-chloropropionyl chloride is 1: 1.1, the mol ratio between chlorobenzene, aluminum trichloride and NaCl is 1: 2.8: 1.1, and the temperature of addition is room temperature, at Stir at 45-65°C for 1 hour, heat up to 150-165°C and react for 1 hour, cool to 80-100°C, pour into 2kg of ice-water hydrochloric acid, filter with suction to obtain 335g of crude product, and obtain 219.5g of solid after recrystallization. The yield is 74% (purity > 98%), and the melting point is 97-98°C.
[0031] HNMR (ppm, CDCl 3) 7.68-7.71 (m, 1H), 7.45-7.49 (m, 1H), 7.36-7.38 (m, 1H), 3.13-3.16 (t, 2H), 2.71-2.74 (t, 2H).
Embodiment 2
[0032] The preparation of embodiment 2.5-bromo-2,3-dihydro-1-indanone
[0033]
[0034] Add bromobenzene (100g, 0.64mol), aluminum trichloride (212.5g, 1.6mol), NaCl (37g, 0.64mol) into a 1-liter three-necked flask, stir, and add 3-chloropropane dropwise at 20-30°C Acyl chloride (83g, 0.65mol), the molar ratio of bromobenzene and 3-chloropropionyl chloride is 1:1, the molar ratio between bromobenzene, aluminum trichloride and NaCl is 1:2.5:1, at 50~70℃ Stir at low temperature for 30 minutes, stir at 160-165°C for 1 hour, cool to 70-80°C, pour into 1kg of ice-water hydrochloric acid, filter with suction to obtain 107g of crude product, and obtain 85g of solid after recrystallization, with a yield of 63% (purity> 98%), melting point 127 ~ 128 ℃.
[0035] HNMR (ppm, CDCl 3 ) 7.59-7.64 (m, 2H), 7.48-7.51 (m, 1H), 3.09-3.12 (t, 2H), 2.66-2.69 (t, 2H).
Embodiment 3
[0036] The preparation of embodiment 3.5-fluoro-2,3-dihydro-1-indanone
[0037]
[0038] Add fluorobenzene (96g, 1mol), aluminum chloride (333g, 2.5mol), NaCl (65g, 1.1mol) in a 1-liter three-necked flask, stir, and add dropwise 3-chloropropionyl chloride ( 133g, 1.05mol), the molar ratio of fluorobenzene and 3-chloropropionyl chloride is 1: 1.1, the molar ratio between fluorobenzene, aluminum trichloride and NaCl is 1: 2.5: 1.1, stirring at 65~70°C 2 hours, stirred at 165-175°C for 3 hours, cooled to 75-80°C, poured into 1kg of ice-water hydrochloric acid, filtered with suction to obtain 134g of crude product, and obtained 84g of solid after recrystallization, with a yield of 56% (purity>98%) ), with a melting point of 38-39°C.
[0039] HNMR (ppm, CDCl 3 ) 7.75-7.78 (m, 1H), 7.57-7.59 (m, 1H), 7.42-7.45 (m, 1H), 3.17-3.20 (t, 2H), 2.85-2.87 (t, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com