Long-chain alkyl sulfoacid functionalized heteropolyacid salt, preparation method and application of long-chain alkyl sulfoacid functionalized heteropolyacid salt
A long-chain alkyl sulfonic acid and heteropoly acid salt technology, applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve problems such as poor catalytic conversion effect, achieve energy saving, simple synthesis process, Effect of High Selectivity and Catalyst Recyclability Performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
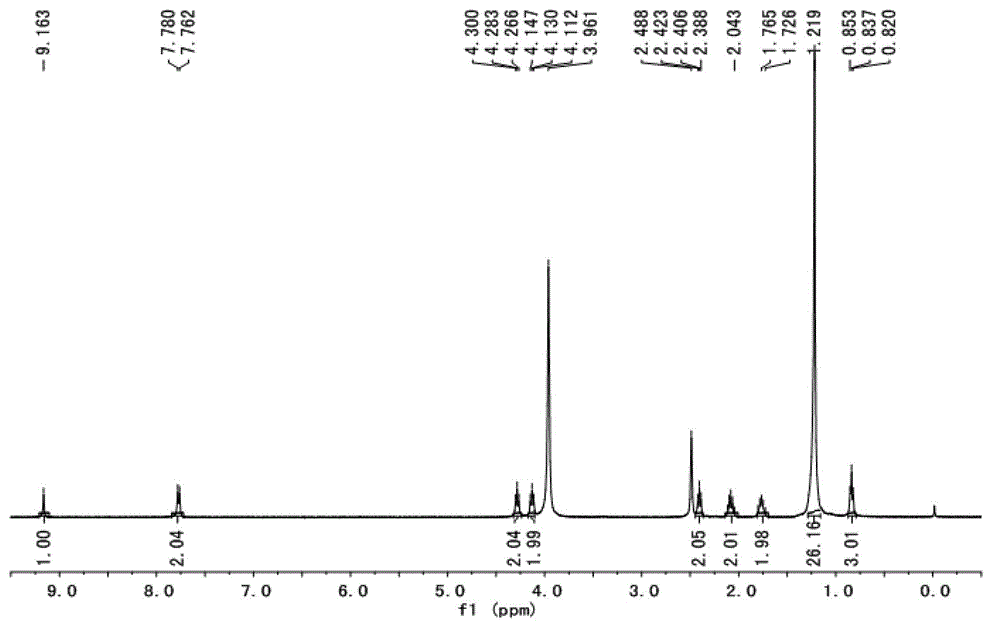
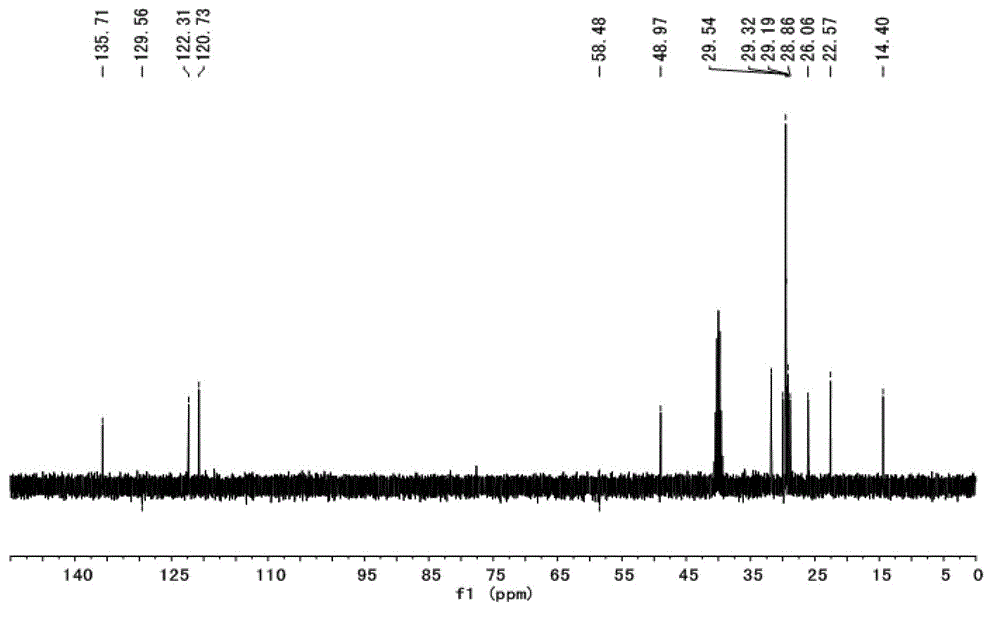
[0035] Embodiment 1: [C1 6 imC 3 SO 3 H] 3 [PW 12 o 40 ] (n=1, m=15, t=3, Y=PW 12 o 40 )Synthesis:
[0036] Under a nitrogen atmosphere, sodium hydride (1.44g, 60mmol) and tetrahydrofuran (100mL) were successively added to a 250mL three-necked flask, and a solution of imidazole (3.45g, 50mmol) in tetrahydrofuran (40mL) was slowly added dropwise at 40°C. After the dropwise addition, continue to stir and react for 4 hours; then increase the reaction temperature to 60°C, slowly drop hexadecane bromide (15.20g, 50mmol) for constant temperature reaction for 12h, and remove the solvent under reduced pressure after the reaction to obtain hexadecyl imidazole , the yield is 98%.
[0037] At 40°C, cetyl imidazole (10.75g, 35mmol) and acetone (60mL) were added to a 250mL three-necked flask, and then 1,3-propane sultone (4.28g , 35mmol) of acetone (60mL) solution, the dropwise addition was completed and the constant temperature reaction was continued for 12h. After the reaction w...
Embodiment 2
[0040] Embodiment 2: [C 16 imC 3 SO 3 H] 3 [PMo 12 o 40 ] (n=1, m=15, t=3, Y=PMo 12 o 4 )Synthesis:
[0041] Under a nitrogen atmosphere, sodium hydride (1.44g, 60mmol) and tetrahydrofuran (100mL) were successively added to a 250mL three-neck flask, and a solution of imidazole (3.45g, 50mmol) in tetrahydrofuran (40mL) was slowly added dropwise at 40°C. After the dropwise addition, continue to stir and react for 4 hours; then increase the reaction temperature to 60°C, slowly drop hexadecane bromide (15.20g, 50mmol) for constant temperature reaction for 12h, and remove the solvent under reduced pressure after the reaction to obtain hexadecyl imidazole , the yield is 98%.
[0042] At 40°C, cetyl imidazole (10.75g, 35mmol) and acetone (60mL) were added to a 250mL three-necked flask, and then 1,3-propane sultone (4.28g ,35mmol) of acetone (60mL) solution, the dropwise addition was completed and the constant temperature reaction was continued for 12h. After the reaction was...
Embodiment 3
[0044] Embodiment 3: [C 16 imC 3 SO 3 H] 4 [SiW 12 o 40 ] (n=1, m=15, t=3, Y=SiW 12 o 40 )Synthesis:
[0045] Add cetyl imidazole (10.75g, 35mmol) and acetone (60mL) into a 250mL three-necked flask at 40°C in 250mL, then slowly add 1,3-propane sultone (4.28 g, 35mmol) of acetone (60mL) solution, the dropwise addition was completed and the constant temperature reaction was continued for 12h. After the reaction was completed, the zwitterionic compound was obtained by filtration with a yield of 95%.
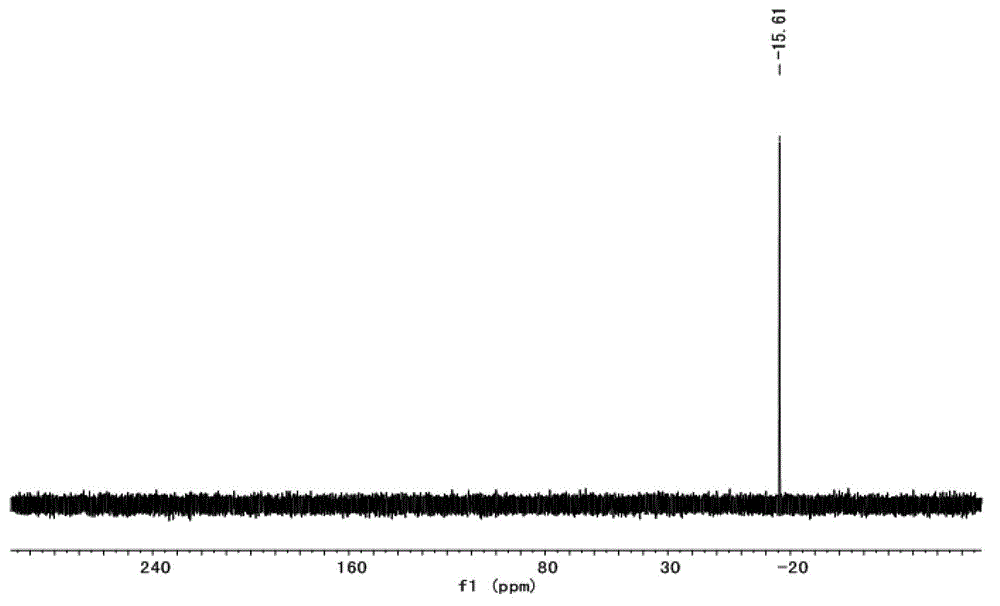
[0046] Take the above-mentioned zwitterionic compound (17.13g, 40mmol) and ethanol (100mL) into a 250mL three-necked flask, and slowly add H 4 SiW 12 o 40 (28.78g, 10mmol) of ethanol (50mL) solution, stirred and reacted for 12h after the dropwise addition, and finally removed the ethanol under reduced pressure to obtain a transparent glassy substance, which is the long-chain alkylsulfonic acid functionalized heteropolyacid salt[ C 16 imC 3 SO 3 H] 4 [SiW 12 o 40 ], ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap