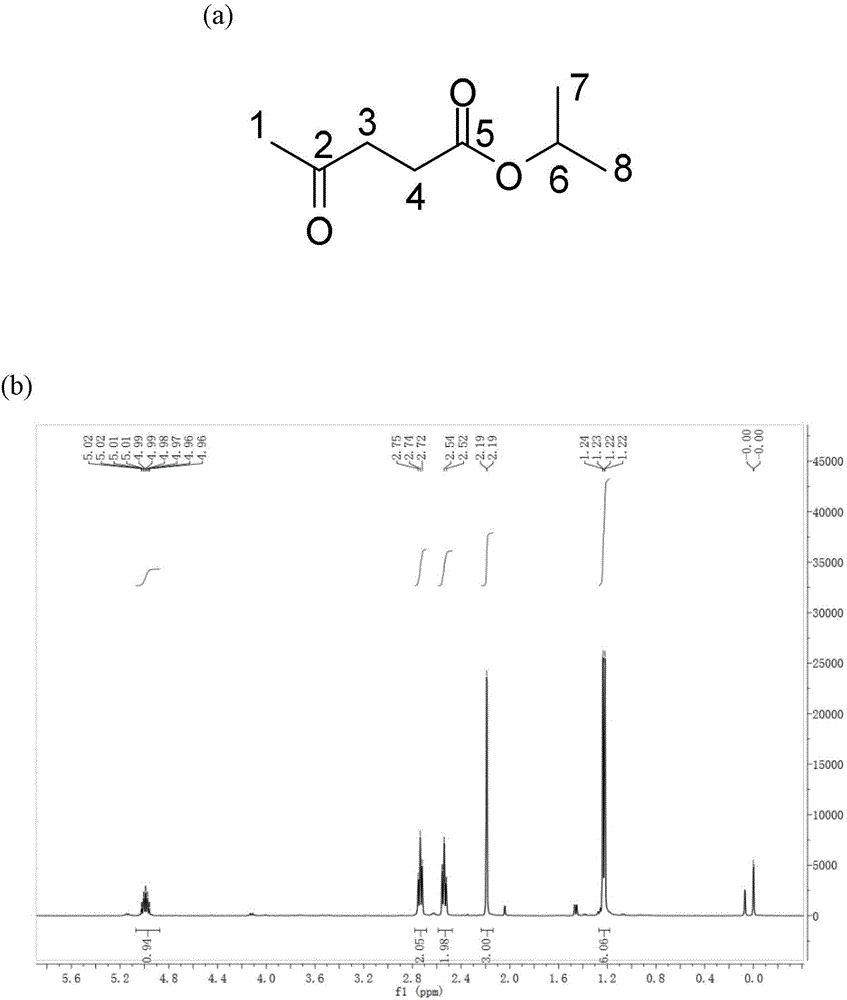
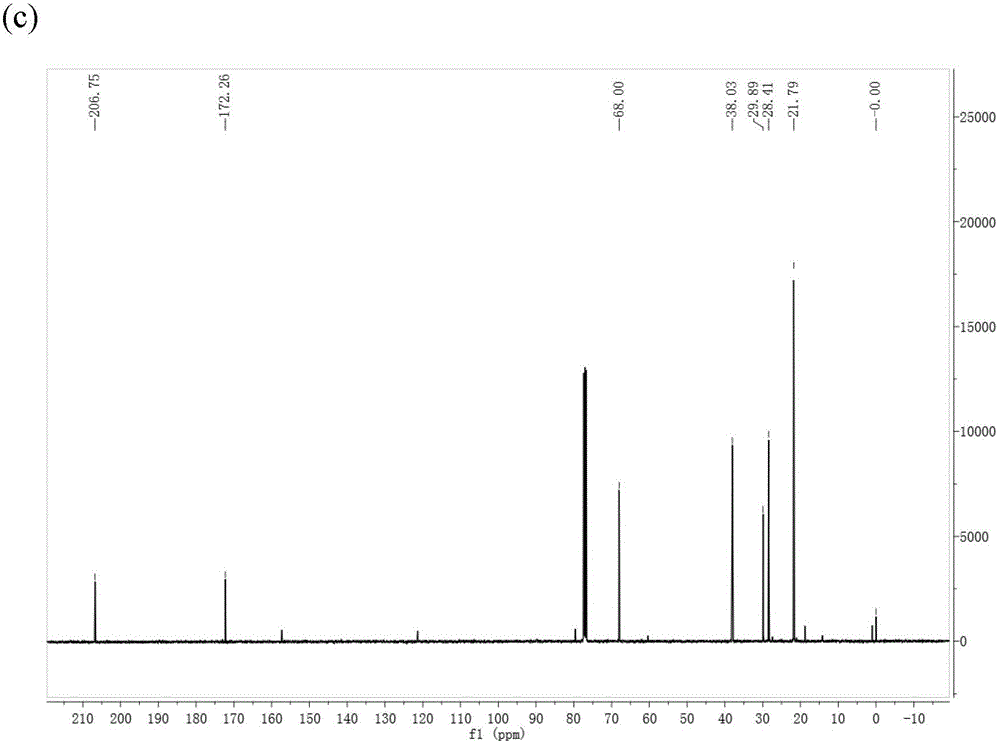
Method for preparing gamma-valerolactone with high selectivity under mild condition
A high-selectivity, valerolactone-based technology, applied in organic chemistry and other fields, can solve problems such as expensive catalysts, long reaction time, and high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Use a dry round-bottomed flask with a branch tube under inert gas N 2 Add 10 mmol of ethyl levulinate and 20 ml of isopropanol under protection, and then add a self-made or commercially available 1 g Raney Ni catalyst (Raney Ni can be purchased from Aldrich and other reagent companies) under inert gas. All operations are performed under inert gas. N 2 Under a protective atmosphere, the flask was closed and reacted at room temperature for 9 hours, and the GVL product was obtained by gas chromatography detection, and a transparent and clear GVL liquid product was obtained by vacuum distillation, and the total yield was 99%.
Embodiment 2
[0065] Put 10 mmol of ethyl levulinate and 20 ml of isopropanol into a dry round-bottomed flask with a branch tube, and then add a homemade or commercially available 1 g Raney Ni catalyst (wet weight), all operations without inert gas protection. , reacted at room temperature for 9 hours, detected by gas chromatography, obtained GVL product, and obtained transparent and clear GVL liquid product through reduced pressure distillation, and the total yield was 70%.
Embodiment 3
[0067] Use a dry round-bottomed flask with a branch tube under inert gas N 2 Add 10 mmol of ethyl levulinate and 20 ml of isopropanol under protection, and then add a homemade or commercially available Raney Ni catalyst with a wet weight of 0.5 g under an inert gas. All operations are performed under an inert gas N 2 Under the protective atmosphere, the flask was closed and reacted at room temperature for 9 hours, and the GVL product was obtained by gas chromatography detection, and the transparent and clear GVL liquid product was obtained by vacuum distillation, and the total yield was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com