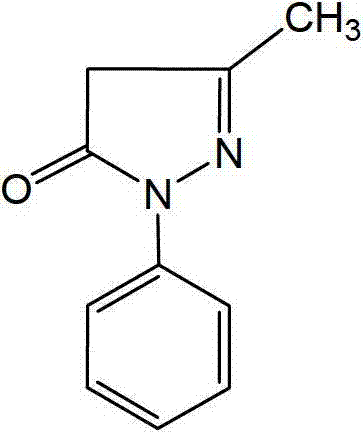
Edaravone injection without antioxidant and preparation method thereof
A technology for edaravone and injection, which is applied in the field of edaravone injection and its preparation, and can solve the problems of harm to human body, influence on product quality, yellowing of medicinal liquid and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The steps of the preparation method of the injection of the present invention are: A, dosing; B, filtering; C, filling; D, sterilizing; in the first 3 steps, nitrogen protection is uniformly filled, and the residual oxygen content in the injection is controlled≤2mg / L.
[0030] The method of the present invention has two kinds of modes when dosing liquid, and one of them adopts heating water for injection, so that dissolve Edaravone. The second is to add acid to adjust the pH value of the aqueous solution to be adjusted to between 1.0-2.0 so as to dissolve the edaravone.
Embodiment 1
[0031] Embodiment 1 adopts heating water for injection to dissolve Edaravone
[0032] prescription:
[0033] Edaravone 30g
[0034] Disodium hydrogen phosphate 10g
[0035] Add water for injection to 20L
[0036] Preparation:
[0037] A. Take 80% water for injection, heat to 80°C, fill with nitrogen, add edaravone, stir until completely dissolved;
[0038] B. Use disodium hydrogen phosphate to adjust the pH value of the solution to 3.0-4.5, and replenish water to the specified scale;
[0039] C. Add activated carbon, stir and absorb for 10 minutes, and filter through a microporous membrane until clarified;
[0040] D. According to 20ml / bottle, fill with nitrogen and sterilize at 121°C for 15 minutes.
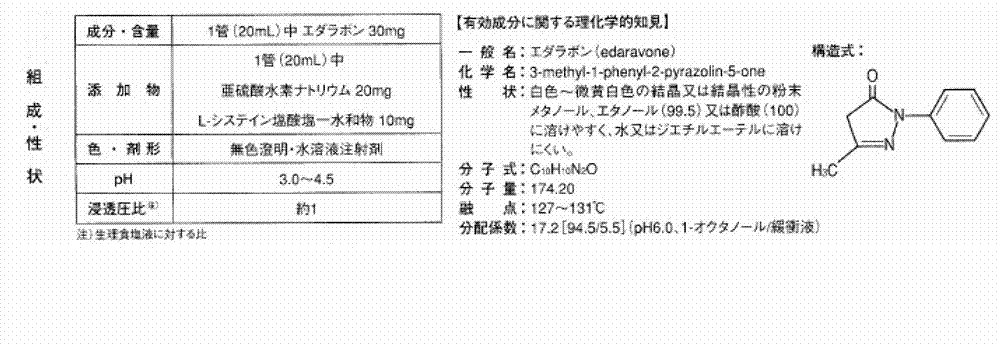
[0041] Comparative Example 1 Japan's commercially available Edaravone injection product instructions are as follows: figure 1 . according to figure 1 The proposed prescription shown is as follows:
[0042]
[0043] Preparation:
[0044] A. Take water for injection ...
preparation example 1
[0109] prescription:
[0110]
[0111] Preparation Process:
[0112] Add 80% water for injection, fill with nitrogen, control the amount of residual oxygen ≤ 2mg / L, keep the water temperature at room temperature (about 25°C), take an appropriate amount of hydrochloric acid solution to adjust the pH value to about 1.5, add edaravone and stir until completely dissolved, and transfer To the water for injection after nitrogen filling, add disodium hydrogen phosphate to adjust the pH value to 3.5-4.5, replenish the water to the specified value, add activated carbon, stir and absorb, filter with 0.22um, 0.45um microporous membrane until clear, and empty the bottle Nitrogen-filled, filled in glass ampoules at 20ml / bottle, filled with nitrogen and then melt-sealed, and sterilized at 121°C for 15 minutes to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com