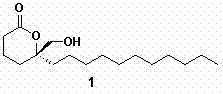
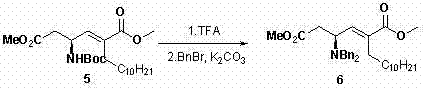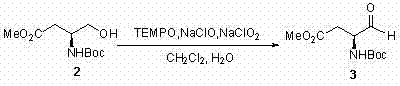Asymmetric full-synthesis method of Tanikolide
A fully synthetic, asymmetric technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve problems that are difficult to meet the needs of in-depth research on chemical properties and biological activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The above-mentioned content of the present invention will be further described in detail below through specific embodiments in the form of examples, but it should not be understood that the scope of the above-mentioned subject of the present invention is limited to the following examples. All technologies realized based on the above contents of the present invention belong to the scope of this invention.
[0030] The following are the embodiments of the present invention, which are divided into ten steps for the convenience of description.
[0031] step 1:
[0032]
[0033] 332 mg (1.43 mmol) primary alcohol 2 Dissolve in 7 mL CH 2 Cl 2 , add 1 mL H 2 O, then add 67 mg (0.43 mmol) TEMPO, 17 mg (0.14 mmol) KBr, 16 mg (0.18 mmol) NaHCO 3 . After stirring at room temperature for 10 min, 1.29 mL (1.7 mmol, 10%) of NaClO was added to the reaction system within 40 min. After the addition, use 100mL CH 2 Cl 2 Dilute the reaction system, successively with 2 × 10 mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com