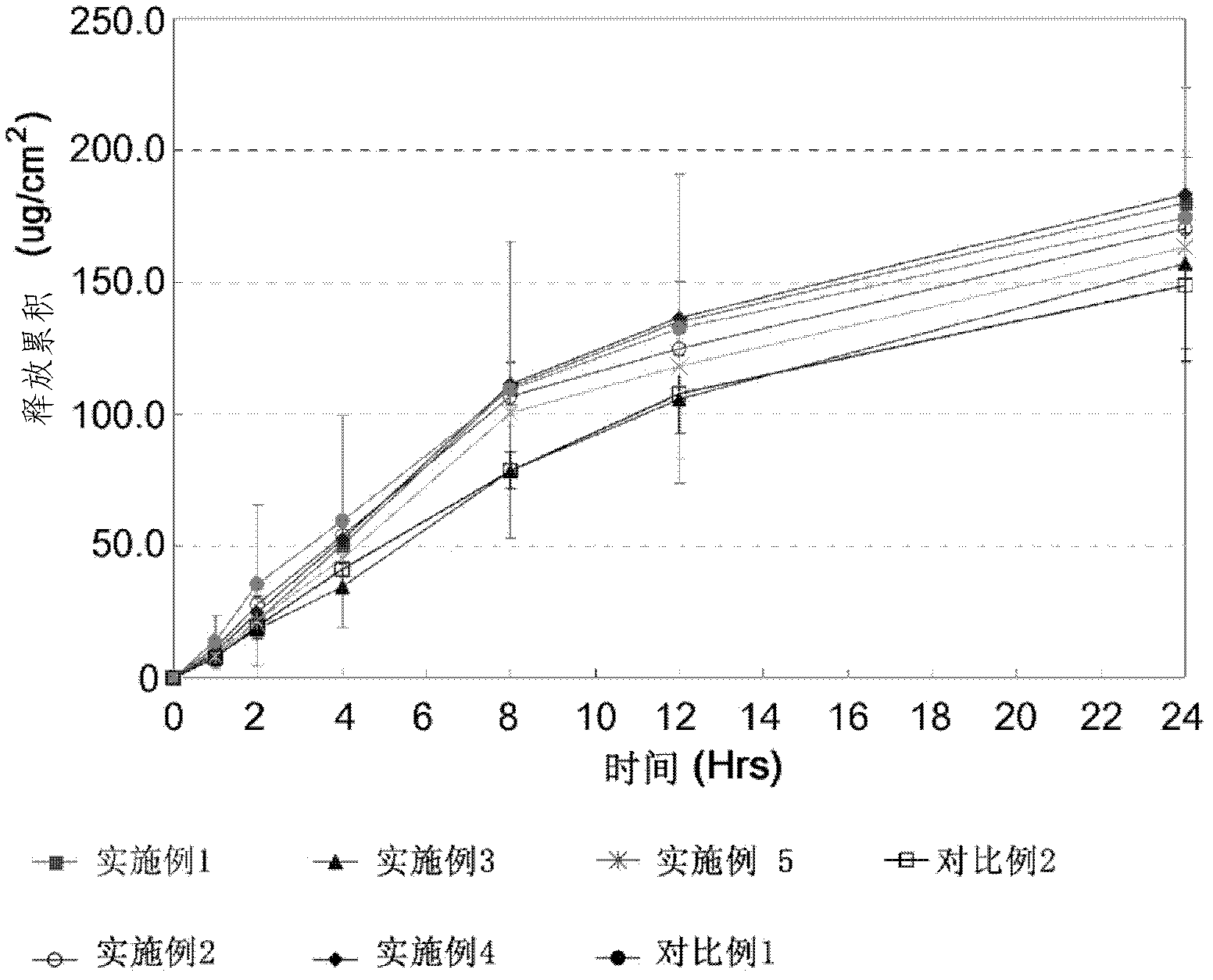
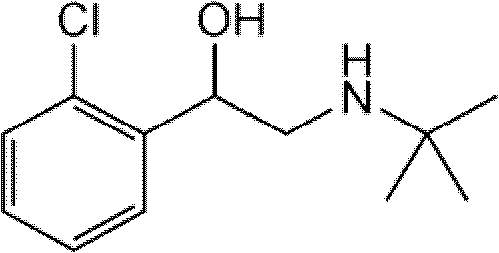
Tulobuterol patch
A technology for adhesives and transdermal absorption preparations, applied in the field of transdermal absorption preparations, can solve problems such as the inability to guarantee consistent release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 2 mg of tulobuterol was dissolved in 5 mg of absolute ethanol, and then 63 mg of acrylate-vinyl acetate adhesive (Duro-Tak 87-2516, Henkel, USA, solid content 41.5%) was added to prepare a drug layer. The drug layer was coated onto a silicon-treated polyethylene terephthalate film as a peelable film using a laboratory coater (Mathis Co. (Mathis Co., Ltd.), Switzerland), It was made to have a dry thickness of 28 μm, and dried at 80° C. for 10 minutes. The film is coated with polyester as a support and rolled into a transdermal formulation.
Embodiment 2
[0035] 2 mg of tulobuterol was dissolved in 5 mg of absolute ethanol, and then 72 mg of acrylate-vinyl acetate adhesive (Duro-Tak 87-2516, Henkel, USA, solid content 41.5%) was added to prepare a drug layer. The drug layer was coated onto a silicon-treated polyethylene terephthalate film as a peelable film with a laboratory coater (Mathis Co. (Mathis Co., Ltd.), Switzerland) to a dry thickness of 32 μm and dried at 80°C for 10 minutes. The film is coated with polyester as a support and rolled into a transdermal formulation.
Embodiment 3
[0037] 2 mg of tulobuterol was dissolved in 5 mg of absolute ethanol, and then 84 mg of acrylate-vinyl acetate adhesive (Duro-Tak 87-2516, Henkel, USA, solid content 41.5%) was added to prepare a drug layer. The drug layer was coated onto a silicon-treated polyethylene terephthalate film as a peelable film with a laboratory coater (Mathis Co. (Mathis Co., Ltd.), Switzerland) to a dry thickness of 35 μm and dried at 80°C for 10 minutes. The film is coated with polyester as a support and rolled into a transdermal formulation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com