The use of protocrotaline in the preparation of anti-asthma medicine
A technology of protomonetine and asthma, applied in the field of protomonitorine in the preparation of anti-asthma drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The structural formula of the compound protobadenine used in the present invention is as follows:
[0024]
[0025] Among the present invention, the mixture preparation method of DRA and aluminum agent is as follows:
[0026] Preparation of the DRA mix :D (m) :R (m) : A (m) =1:10:1, dissolved in PBS, the concentration is 6mg / ml, mixed with the aluminum agent at a volume ratio of 1:19, and mixed on a suspension apparatus at 4°C for 4-5 hours to obtain DRA and aluminum agent mixture.
[0027] DRA mixture formulation for intranasal administration: D (m) :R (m) : A (m) =1:10:1, dissolved in PBS, the concentration is 2mg / ml.
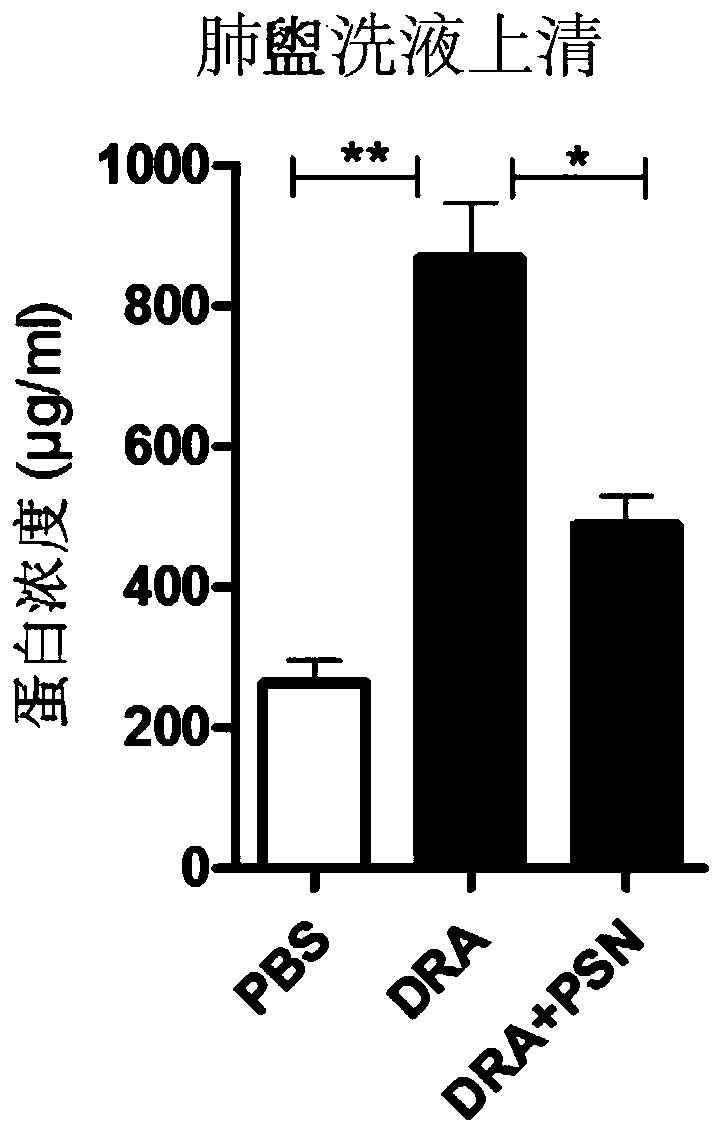
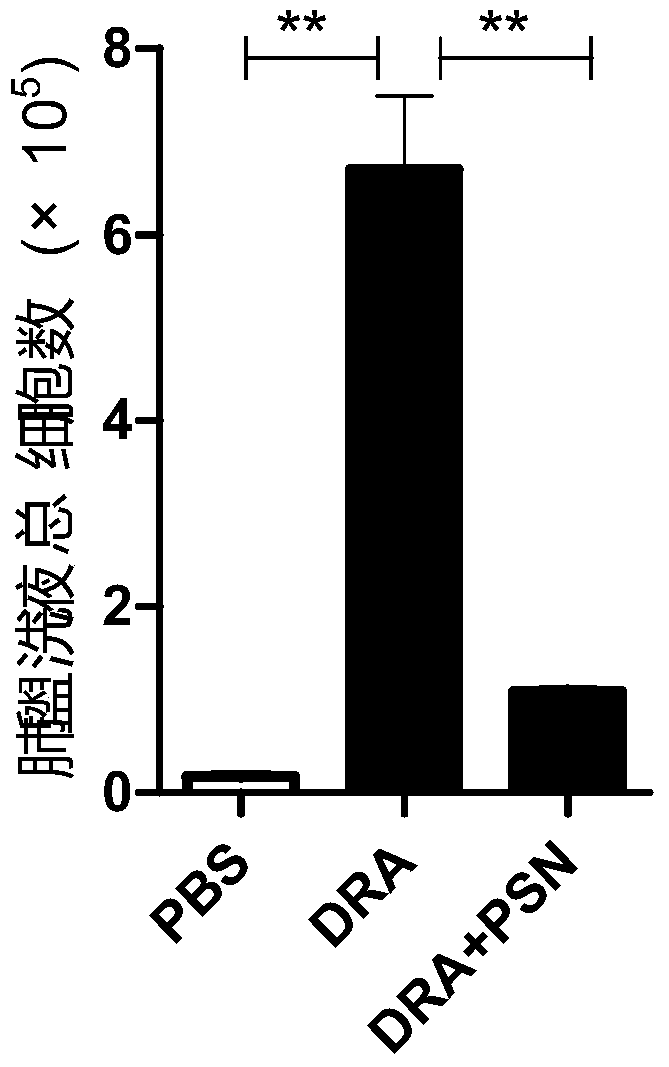
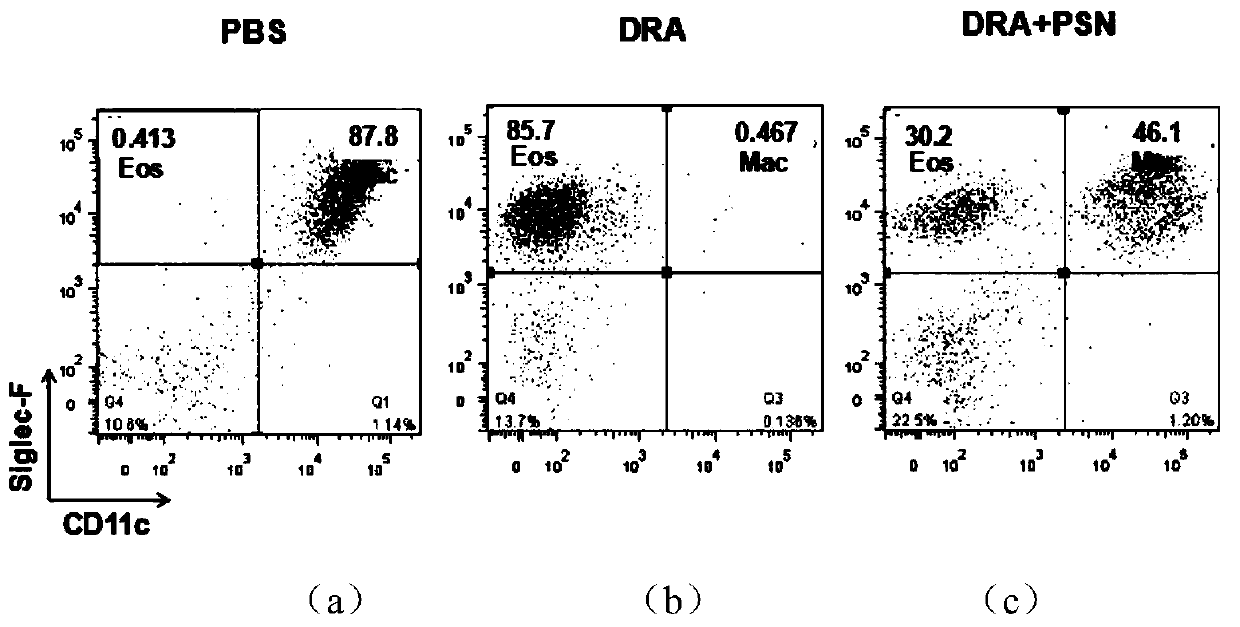
[0028] In the present example, through animal experiments, it was found that protobarkine has a significant therapeutic effect on asthma, and the experimental methods and results are as follows:
[0029] 1 Experimental materials
[0030] 1.1 Experimental animals:
[0031] C57 / BL mice, male, weighing 20-25 g, were purchased from the Experim...
Embodiment 2
[0057] Preparation of tablets:
[0058] Take 300g of protomonetine, add appropriate amount of starch to make granules, add appropriate amount of magnesium stearate, mix well, press into 1000 tablets for oral administration, 2 times a day, 2 tablets each time.
Embodiment 3
[0060] Preparation of capsules:
[0061] Take 300g of protomonetine, add appropriate amount of diluent, mix well, divide into empty capsules and get final product. Make 1000 capsules, 2 times a day, 2 capsules each time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com