Medicine clinical test supervision and administration system
A technology for clinical trials and supervision and management, applied in data processing applications, instruments, calculations, etc., can solve the problems of manual supervision, such as labor-intensive, untrue, and large R&D capital turnover pressure, and achieve the effect of improving supervision efficiency and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
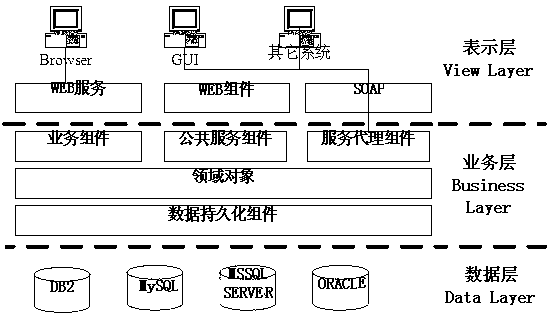
[0015] The drug clinical trial supervision and management system of the present invention is described in detail below in conjunction with the accompanying drawings and specific embodiments:
[0016] The drug clinical trial supervision and management system of the present invention includes four parts: institutional supervision of participating clinical trial projects, project process supervision, adverse event supervision and trial drug supervision, and each supervision part is composed of a presentation layer, a business layer and a data layer. The presentation layer includes WEB servers, WEB components and SOAP (Simple Protocol Objects). The business layer includes business components, public service components, service proxy components and domain objects. The data layer is the underlying database structure. The JPA framework is used to standardize the data. In the architecture, the annotation-based JPA ORM is used to map the tables in the database, and the information of e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com