Synthesis method for N-(3-aminopropyl) methacrylamide hydrochloride
A technology of methacrylamide hydrochloride and methacryloylpropylenediamine, which is applied in the field of synthesis of N-methacrylamide hydrochloride and can solve the problem of N-(3-aminopropyl)methyl Acrylamide hydrochloride synthesis reports and other issues, to achieve the effect of high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
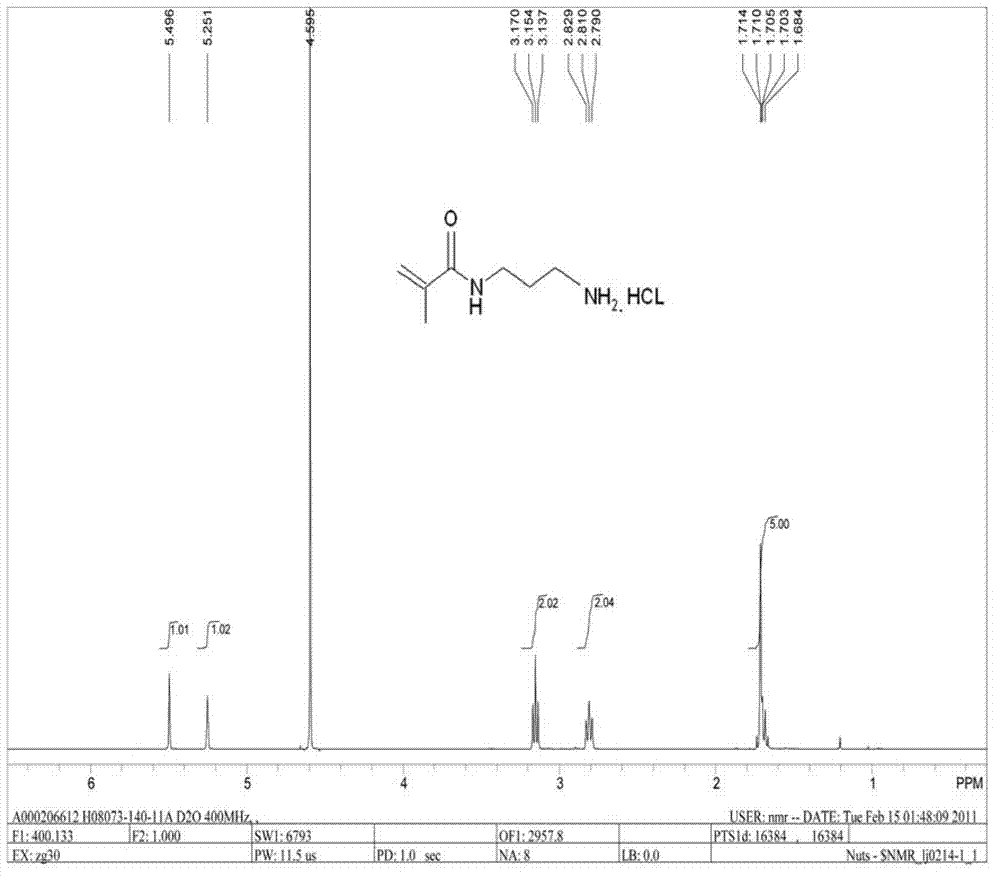
[0028] The synthetic method of N-(3-aminopropyl) methacrylamide hydrochloride,
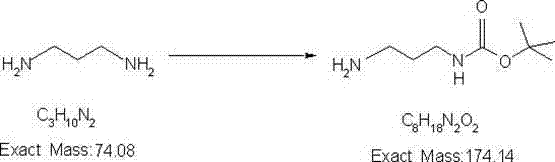
[0029] first step:
[0030]
[0031] Dissolve 100 grams of propylenediamine in 1000 milliliters of ethyl acetate, lower the temperature to below 0°C, dissolve 147 grams of di-tert-butyl dicarbonate in 500 milliliters of ethyl acetate and add dropwise, and keep reacting during the dropping process liquid below 10°C. After the dropwise addition, the reaction was completed for 10 hours, and the reaction was detected by GC. Ethyl acetate was distilled off under normal pressure, excessive propylenediamine was distilled off under the circulating water pump, and then 50 grams of N-Boc-1.3-propylenediamine was distilled under the vacuum condition of 5mm / hg, yield 22%, GC Purity greater than 95% for the next reaction.
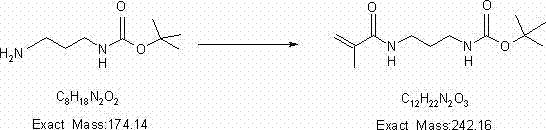
[0032] Step two:
[0033]
[0034] Dissolve 50 grams of N-Boc-1.3-propylenediamine prepared in the first step in 500 milliliters of ethyl acetate, add 34.8 grams of triethylamine,...
Embodiment 2
[0039] first step:
[0040]
[0041] Dissolve 100g of propylenediamine in 1000ml of ethyl acetate, cool down to below 0°C, dissolve 98g of di-tert-butyl dicarbonate in 330ml of ethyl acetate and add dropwise, keep the reaction solution at 10°C during the dropwise addition the following. After the dropwise addition, the reaction was completed for 10 hours, and the reaction was detected by GC. Ethyl acetate was distilled off under atmospheric pressure, excessive propylenediamine was distilled off under the circulating water pump, and then 47g of N-Boc-1.3-propylenediamine was distilled under vacuum conditions with a vacuum degree of 1mm / hg, the yield was 20%, and the GC purity was greater than 95% was used for the next reaction.
[0042] Step two:
[0043]
[0044] Dissolve 47g of N-Boc-1.3-propylenediamine prepared in the first step in 900ml of ethyl acetate, add 27g of triethylamine, lower the temperature below 0°C, add 28g of methacryloyl chloride dropwise, a...
Embodiment 3
[0049] first step:
[0050]
[0051] Dissolve 100g of propylenediamine in 1200ml of ethyl acetate, cool down to below 0°C, dissolve 294g of di-tert-butyl dicarbonate in 800ml of ethyl acetate and add dropwise, keep the reaction solution at 10°C during the dropwise addition the following. After the dropwise addition, the reaction was completed for 10 hours, and the reaction was detected by GC. Ethyl acetate was distilled off at normal pressure, excess propylenediamine was distilled off under the circulating water pump, and then 35g of N-Boc-1.3-propylenediamine was distilled under vacuum conditions with a vacuum degree of 600mm / hg, the yield was 15%, and the GC purity was greater than 95 % for the next reaction.
[0052] Step two:
[0053]
[0054] Dissolve 35g of N-Boc-1.3-propylenediamine prepared in the first step in 200ml of ethyl acetate, add 40g of triethylamine, lower the temperature below 0°C, add 31g of methacryloyl chloride dropwise, and keep the temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com