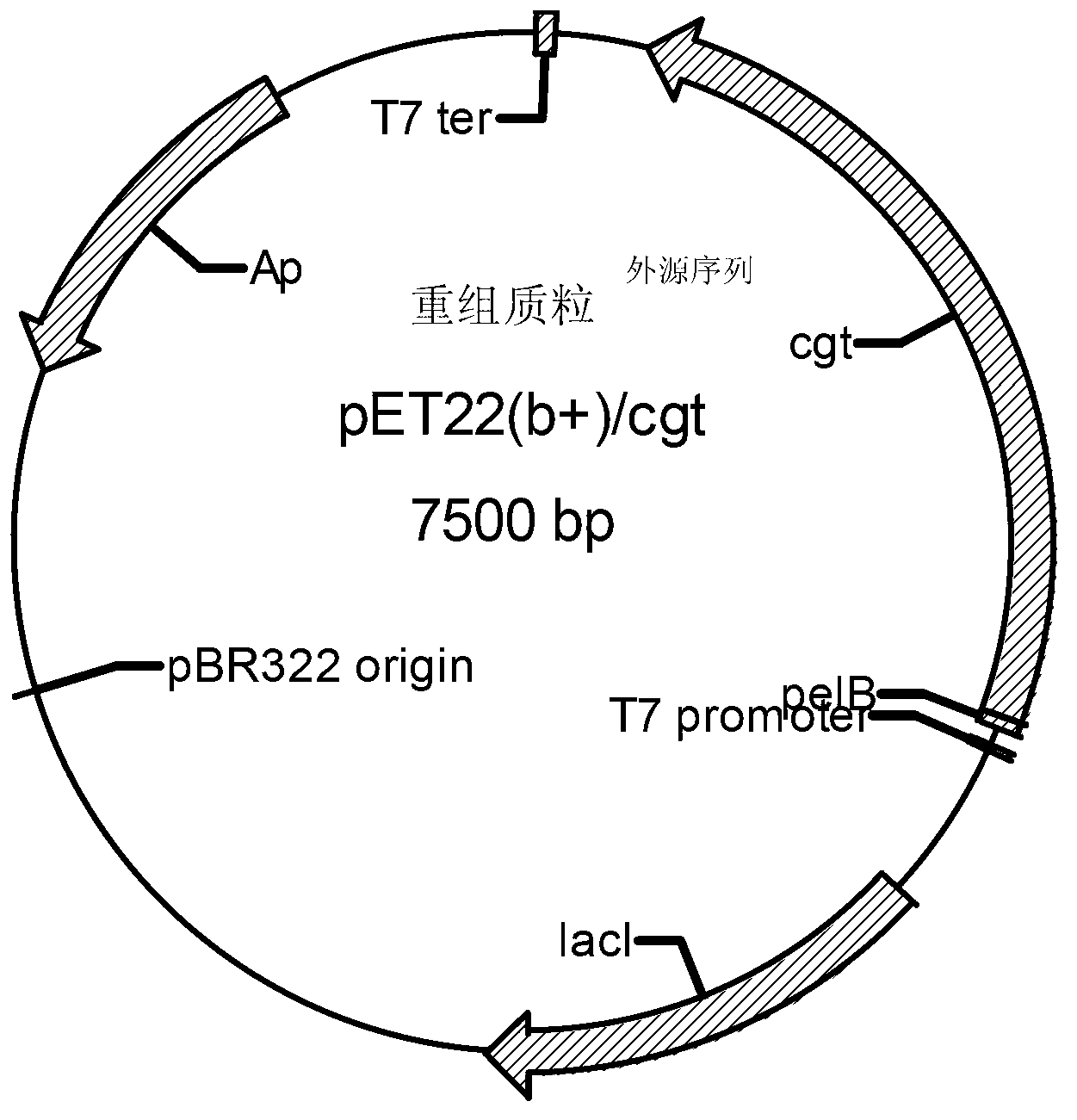
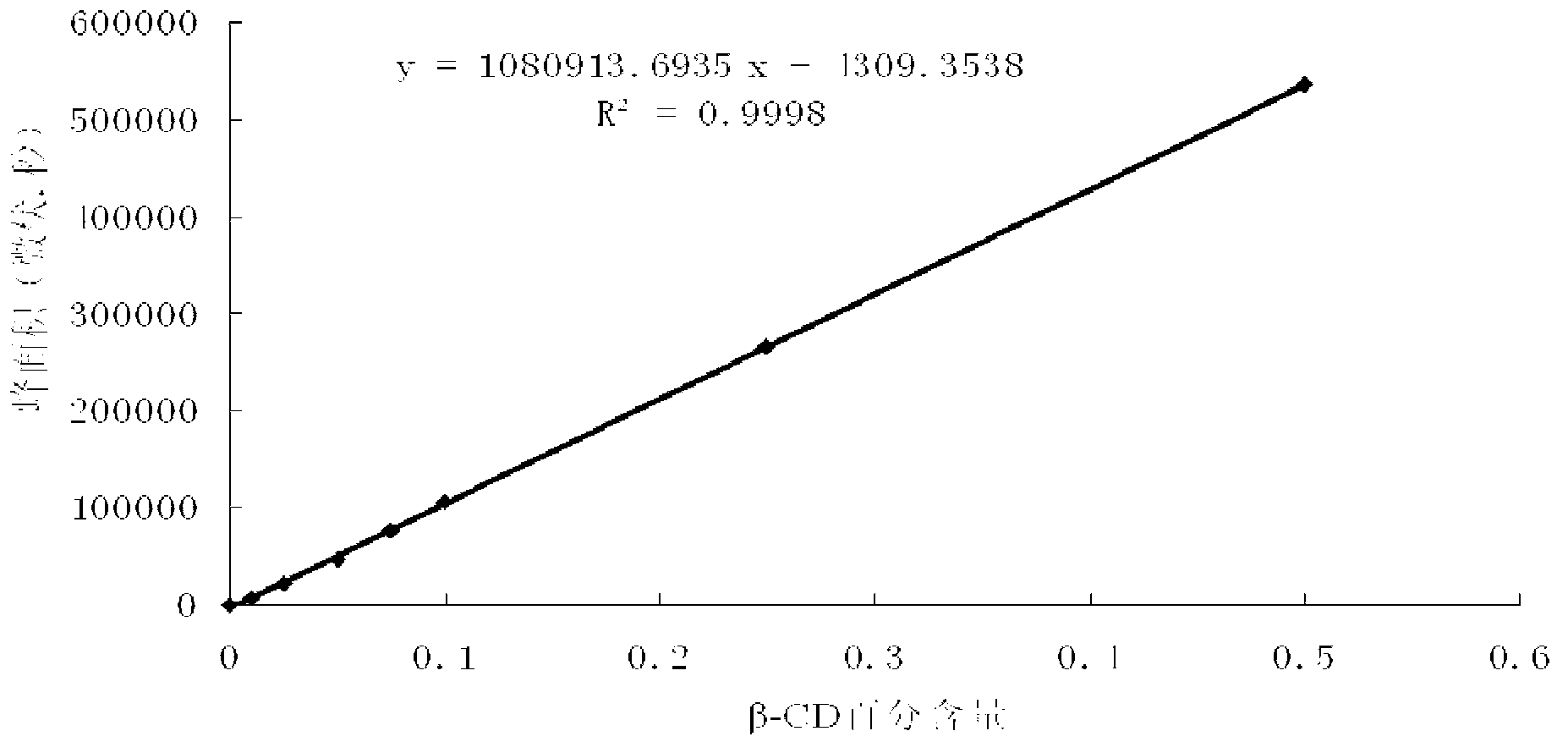
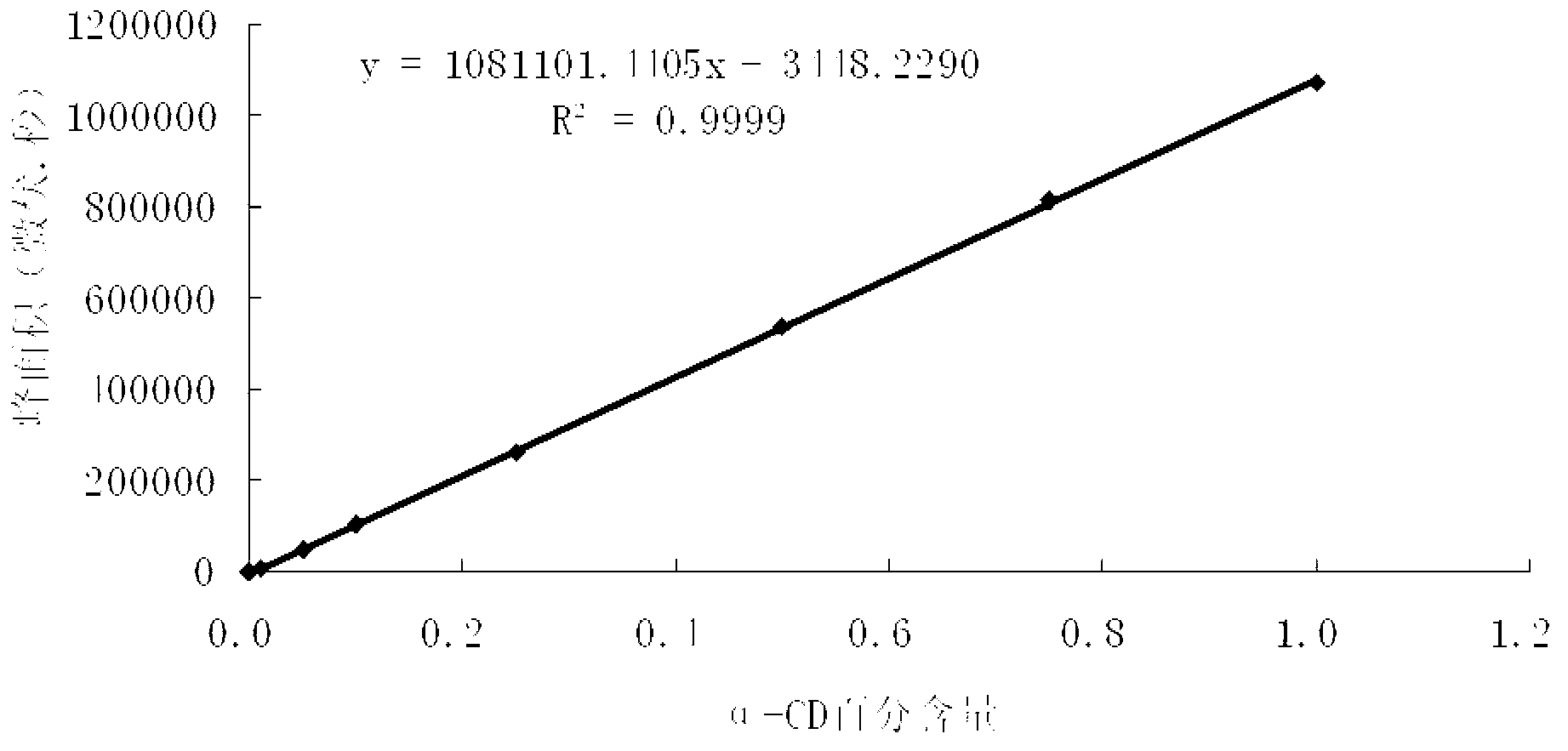A group of cyclodextrine glucosyltransferases and encoding gene and application thereof
A cyclodextrin and gene technology, applied in the directions of transferase, application, genetic engineering, etc., can solve the problems of high price, small production scale, limitation of γ-cyclodextrin production and application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1, the discovery of mutated protein and its coding gene
[0071] A strain of Bacillus macerans screened from nature can produce cyclodextrin glycosyltransferase, and the cyclodextrin glycosyltransferase gene in the strain is shown in sequence 2 of the sequence listing, encoding sequence 1 of the sequence listing proteins indicated.
[0072] The protein shown in Sequence 1 is named α-CGTase-1 protein, and the gene encoding it is named α-CGTase-1 gene (as shown in Sequence 2). The protein shown in Sequence 3 was named α-CGTase-WT protein (sequence published in NCBI), and the gene encoding it was named α-CGTase-WT gene (shown in Sequence 4).
[0073] The difference between Sequence 1 and Sequence 3 lies only in the 590th amino acid residue (M / V) and the 677th amino acid residue (S / G). The difference between sequence 2 and sequence 4 lies in the 1768th nucleotide (A / G) and the 2029th nucleotide (A / G).
[0074] Using the double-stranded DNA shown in Sequence 1 ...
Embodiment 2
[0075] Embodiment 2, enzyme activity identification
[0076] 1. Artificially synthesize the following double-stranded DNA molecules:
[0077] (1) Double-stranded DNA molecule 1: The difference from the DNA molecule shown in Sequence 2 of the sequence table is that the 583-585 nucleotides (TAC) from the 5' end will be deleted, and the 499-501 nucleotides from the 5' end will be deleted. The nucleotide at position 1 is mutated from TAC to CAC; correspondingly, the 195th tyrosine in sequence 1 is deleted, and the 167th amino acid residue is mutated from tyrosine to histidine. The protein encoded by double-stranded DNA molecule 1 is named protein 1.
[0078] (2) Double-stranded DNA molecule 2: The difference from the DNA molecule shown in Sequence 2 of the sequence table is that the 583-585 nucleotides from the 5' end are mutated from TAC to TTC, and the 499- The 501st nucleotide is mutated from TAC to CAC; correspondingly, the 195th amino acid residue in sequence 1 is mutated f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com