Thalassemia gene detection method based on fluorescence labeling quantitation PCR (Polymerase Chain Reaction) technology
A fluorescent labeling quantitative, thalassemia technology, applied in the field of medical detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: detection method of the present invention
[0084] (1) Primer design
[0085] (1) Through the Blast function of NCBI, design a DNA fragment with a length of 20 bp and less homology with human DNA sequences as a universal primer. The sequence of the universal primer is: 5'-CTCGACACGCATCTGCTCAG-3' (SEQ ID NO: 1) , using 6-FAM fluorescent labeling at the 5' end to obtain universal fluorescent labeling primers;
[0086] In addition, 5'-CTCGACACGCATCTGACGTT-3' (SEQ ID NO: 2) labeled with VIC, 5'-CTCGACACGCATCTGGCGAA-3' (SEQ ID NO: 3) labeled with NED, and 5'- CTCGACACGCATCTGCTACC-3' (SEQ ID NO: 4) has also been designed and can be used in subsequent multicolor fluorescent labeling systems;
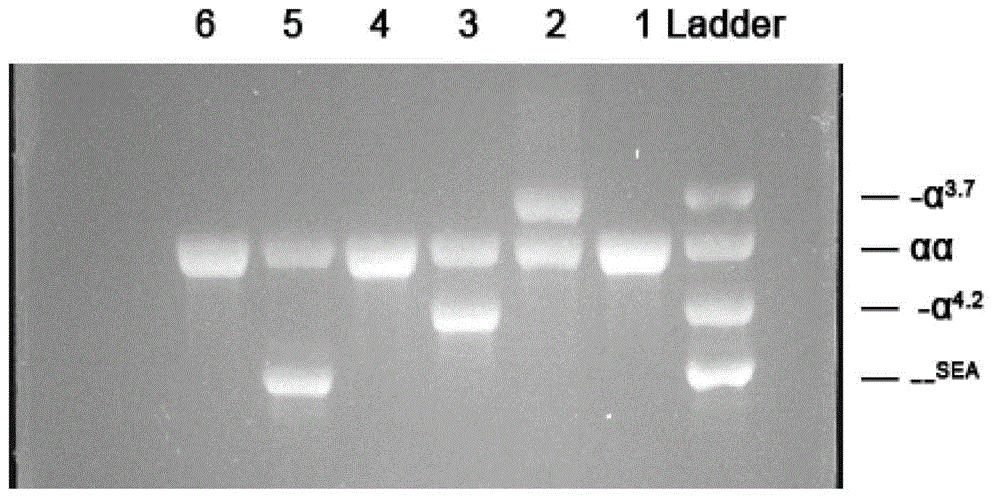
[0087] (2) For α-thalassemia -- SEA Type, -α 3.7 ,-α 4.2 ,-- THAI , α CS alpha, alpha QS α and α WS α, and β thalassemia HPFH-SEA and DBT thalassemia genotype sequences, design amplification primer pairs, primer sequences and pairings are as follows:
[0088] 2.1)...
Embodiment 2
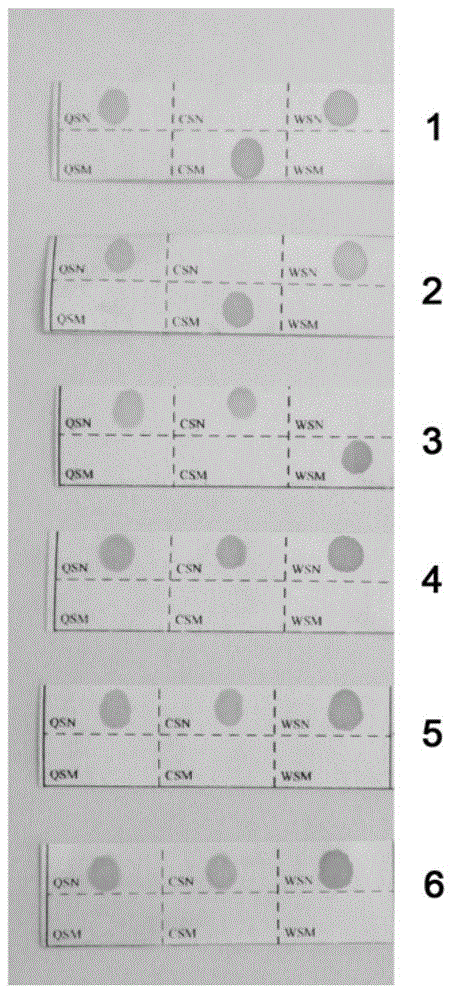
[0189] Repeat the method of embodiment 1, difference is, replace sample 1 with sample 2, the result is as follows Figure 6 shown.
[0190] Figure 6 Among them, 2Y1=Y2, CSM, QSN, WSN have peaks, HPFH-W has peaks, and the rest have no peaks.
[0191] Combining with existing test results Figure 1~4 , it can be independently judged that the genotype of sample 2 is -α 3.7 / α CS α genotype thalassemia; and the result graph independently made by the system of the present invention can also directly determine that the genotype of sample 2 is -α 3.7 / α CS Alpha genotype thalassemia. The two are mutually verified, which proves the simplicity of the present invention and the consistency of the results.
Embodiment 3
[0193] Repeat the method of embodiment 1, difference is, replace sample 1 with sample 3, the result is as follows Figure 7 shown.
[0194] Figure 7 Among them, Y1=2Y2, CSN, QSN, and WSW have peaks, HPFH-W has peaks, and the rest have no peaks.
[0195] Combining with existing test results Figure 1~4 , it can be independently judged that the genotype of sample 3 is -α 4.2 / α WS α genotype thalassemia; and the result graph independently made by the system of the present invention can also directly determine that the genotype of sample 3 is -α 4.2 / α WS Alpha genotype thalassemia. The two are mutually verified, which proves the simplicity of the present invention and the consistency of the results.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com