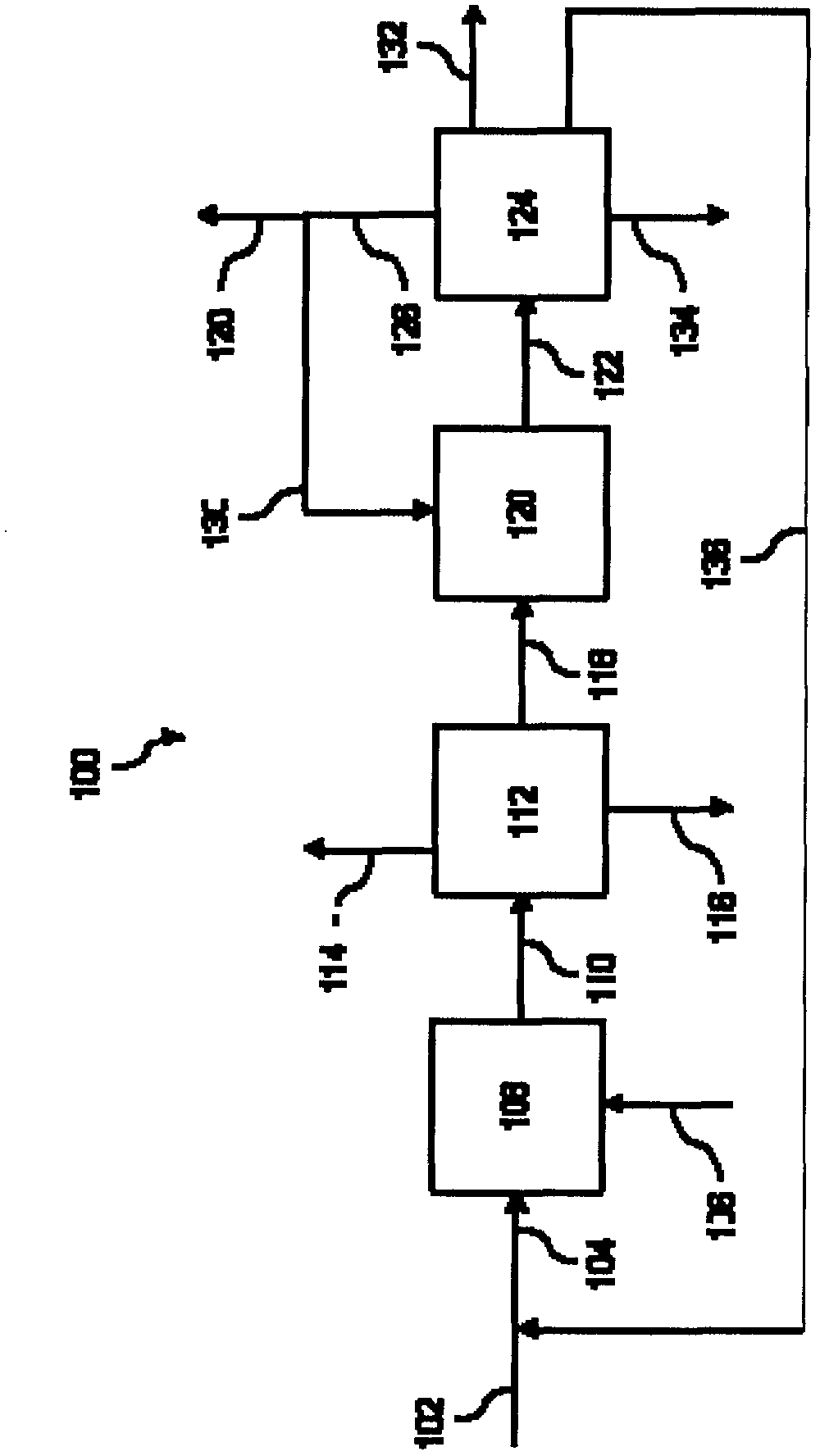
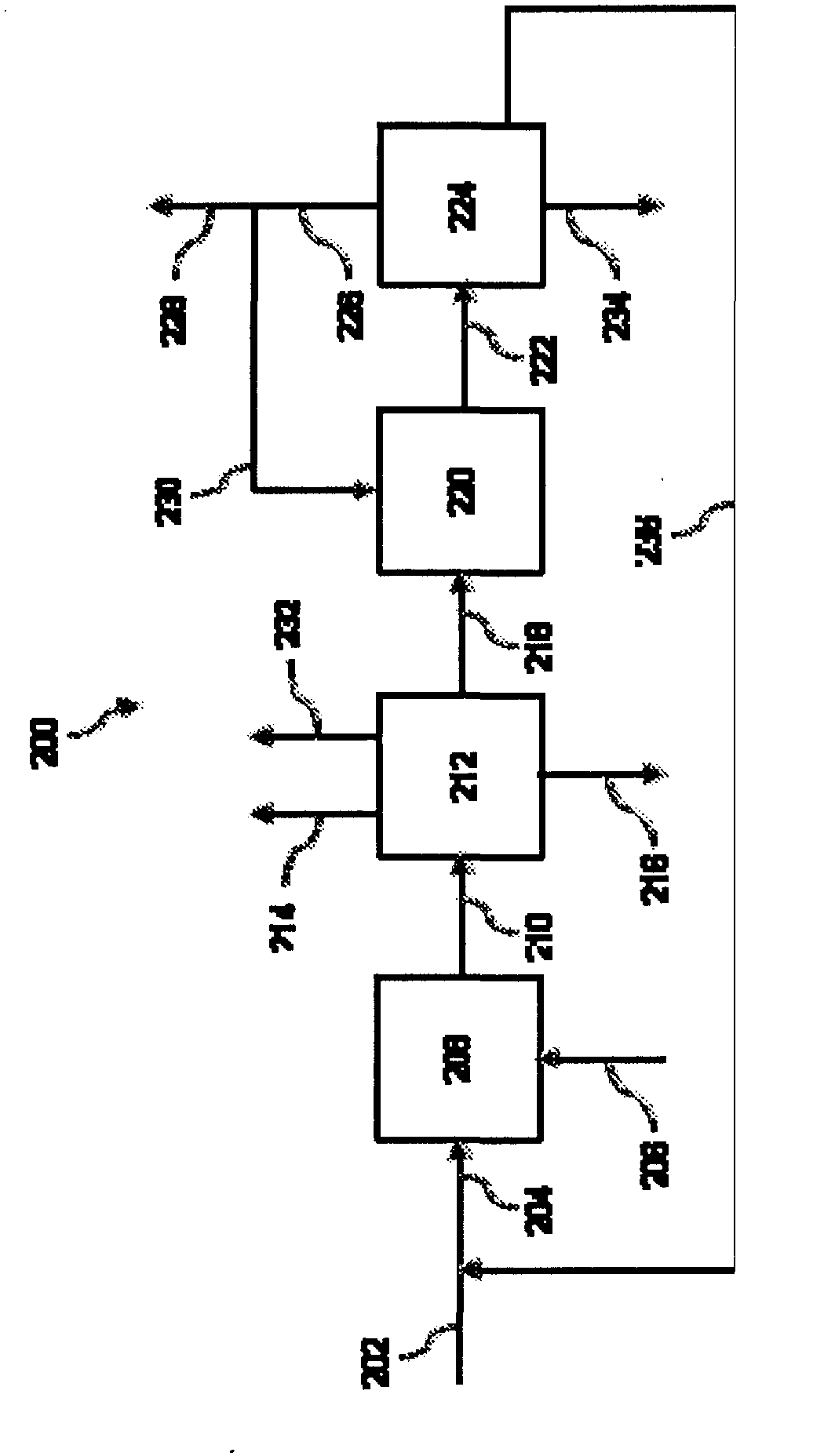
Process of producing cyclohexylbenzene
A technology of cyclohexylbenzene and cyclohexane, which is applied in the field of production of cyclohexylbenzene, can solve the problems of increasing the production of unwanted by-products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
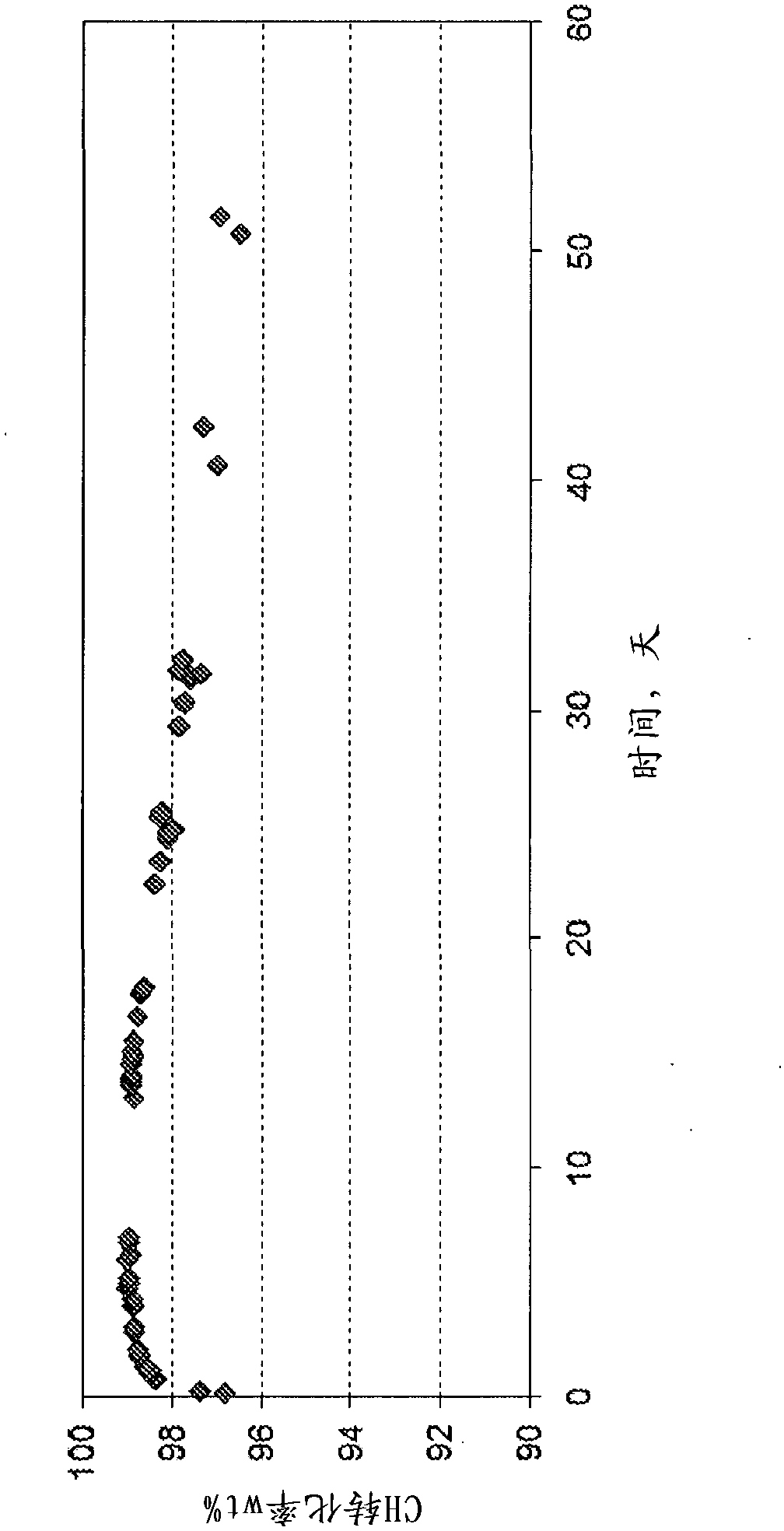
[0129] A dehydrogenation catalyst containing 1 wt% platinum and 0.15 wt% tin on a silica support was crushed to 60 / 100 mesh and loaded into a 1 / 2'' (1.27 cm) outer diameter (OD) tubular downflow reactor Inside. The catalyst was then mixed with a feedstock composition containing 89 wt% benzene, 10 wt% cyclohexane and 1 wt% methylcyclopentane (simulating a portion of the first effluent stream) at 480°C, 0.689MPag, 10hr -1The weight hourly space velocity (WHSV) and the molar ratio of hydrogen to hydrocarbons (H2 / HC) are contacted under the dehydrogenation conditions of 4. Continuous operation of the dehydrogenation reaction was maintained in this manner for more than 50 days, and the dehydrogenation product (second effluent stream) was flashed at ambient temperature (about 25° C.) and from time to time on an Agilent 5690 gas chromatograph, using DB- 1 column, analyzing the liquid fraction. Figure 3-5 The results are summarized in .
[0130] Also in the dehydrogenation product...
Embodiment 2
[0132] Following the long-term experiment of Example 1 (ie starting at about day 53), the dehydrogenation conditions were varied over the course of several days using the same dehydrogenation catalyst on the same apparatus with the same feedstock composition and experimental protocol. The results are reported in Table 1, which demonstrate, inter alia, very low levels of dehydrogenation catalyst activity, and conditions affecting methylcyclopentane. Negative MCP conversion entries indicate an increase in methylcyclopentane content in the dehydrogenation product (second effluent stream) relative to the feedstock composition (simulating a portion of the first effluent stream), which at some These dehydrogenation conditions are possible, especially at lower temperatures. This implies that the acidity of the catalyst is low enough to prevent the destruction of methylcyclopentane to acyclic paraffins, but may exhibit a certain acidity to allow trace amounts of cyclohexane to isomeri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com