Vinylidene-fluoride-based copolymer and application of said copolymer
一种二氟乙烯、类共聚物的技术,应用在1领域,能够解决金属箔粘接性不充分等问题,达到剥离强度优异、粘接性优异、生产率良好的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
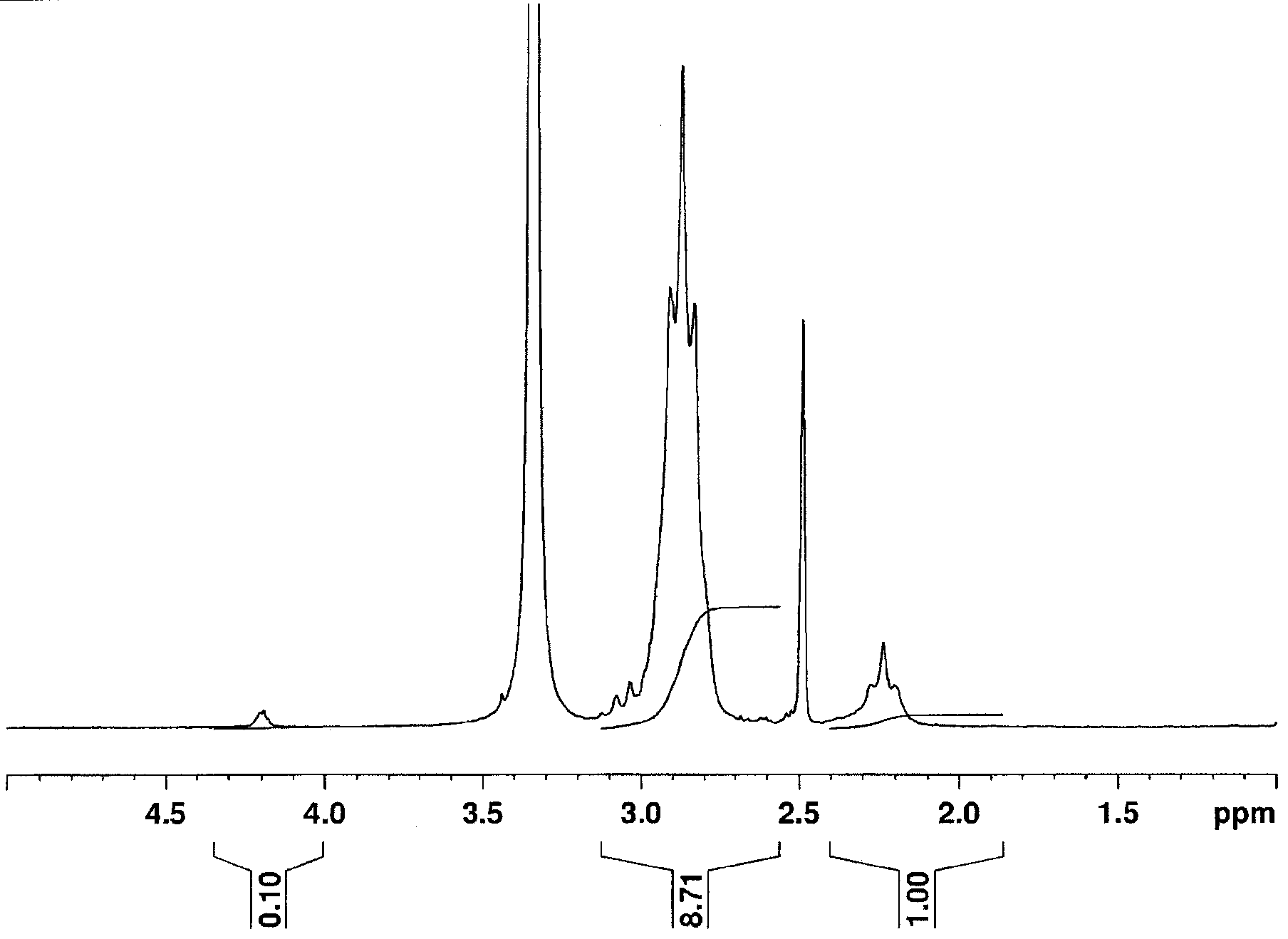
[0145] Polymerization was carried out by the following method to obtain a 1,1-vinylidene fluoride copolymer in the form of polymer powder.
[0146] Into an autoclave with an inner capacity of 2 liters, 900 g of ion-exchanged water, 0.4 g of Metolose 90SH-100 (manufactured by Shin-Etsu Chemical Co., Ltd.), 0.2 g of carboxyethyl acrylate, and 50% by weight of peroxide were charged into an autoclave with an inner capacity of 2 liters. 2.0 g of tert-butyl valerate-Flon 225cb solution and 396 g of 1,1-difluoroethylene were heated to 50° C. over 2 hours.
[0147] Thereafter, while maintaining 50° C., a 15 g / l carboxyethyl acrylate aqueous solution was slowly added at a constant polymerization pressure. A total of 4.0 g of carboxyethyl acrylate was added including the amount added initially.
[0148] The polymerization was stopped at the same time as the addition of the carboxyethyl acrylate aqueous solution was completed, and the temperature was raised for a total of 8.6 hours.
...
Embodiment 2、3
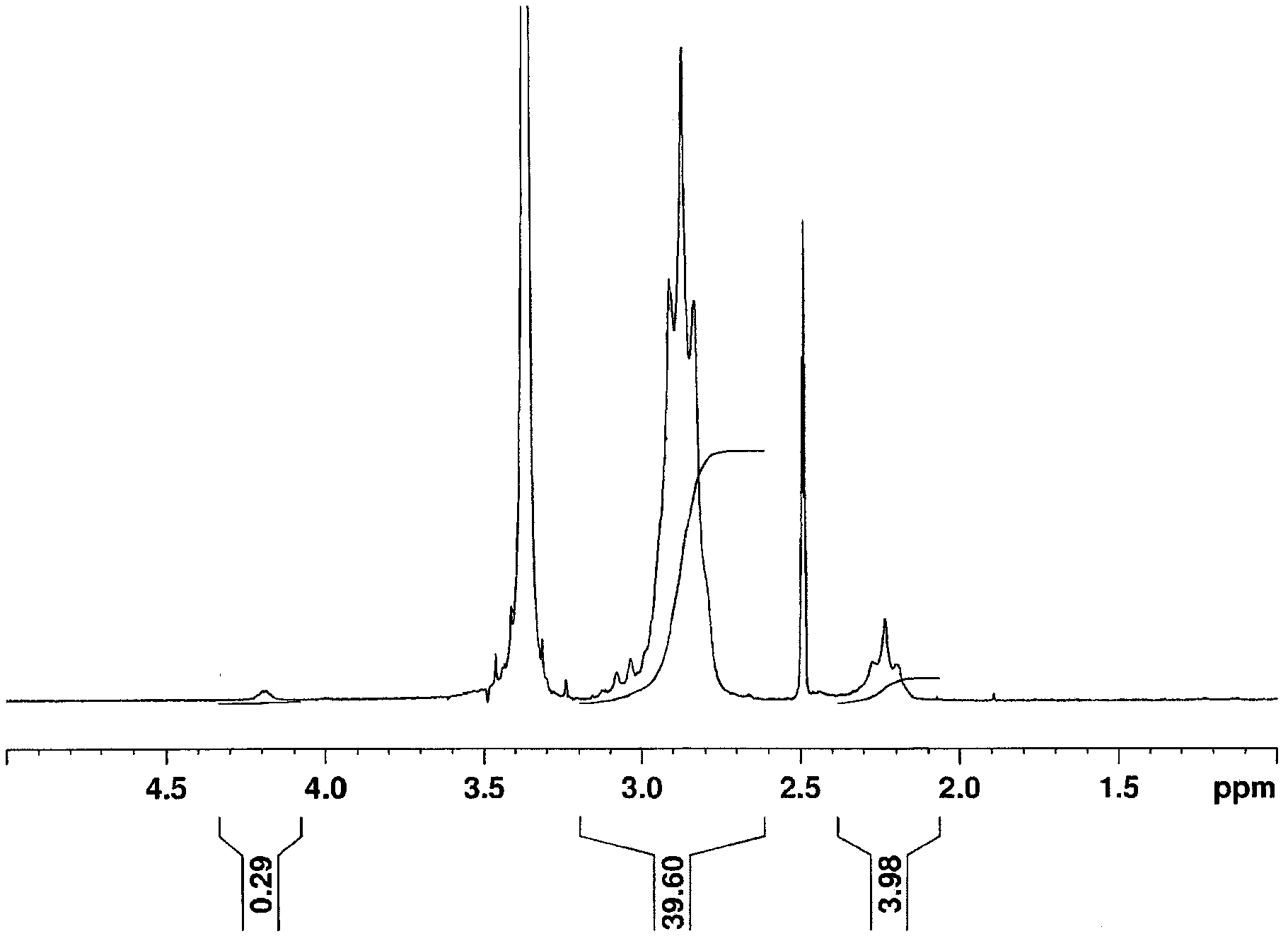
[0167] Except changing the concentration and addition amount of the carboxyethyl acrylate aqueous solution, the addition amount of the initiator, and the polymerization conditions as shown in Table 1, it was performed in the same manner as in Example 1 to obtain a polymer powder. For the yield of polymer, embodiment 2 is 71%, embodiment 3 is 25%, for the inherent viscosity of gained polymer, embodiment 2 is 3.15dl / g, embodiment 3 is 2.65dl / g, For the absorbance ratio of the obtained polymer (A R ), embodiment 2 is 0.70, and embodiment 3 is 2.57.
[0168] Measure the content of above-mentioned polymer powder with the method identical with embodiment 1 1 H NMR spectrum and 19 F NMR spectrum. Same as Example 1 by 1 The integrated intensity of the H NMR spectrum was used to obtain the VDF amount and the CEA amount of the polymer. In addition, similarly to Example 1, by 19 The amount of polymer chains derived from carboxyethyl acrylate was determined from the F NMR spectrum, ...
Embodiment 2
[0169] The 1,1-difluoroethylene-based copolymer obtained in Example 2 had a VDF amount of 99.39 mol%, a CEA amount of 0.61 mol%, and an amount of polymer chains derived from carboxyethyl acrylate of 0.38 mol%. The yield was 62%, the VDF amount of the 1,1-difluoroethylene-based copolymer obtained in Example 3 was 97.28 mol%, the CEA amount was 2.72 mol%, and the amount of polymer chains derived from carboxyethyl acrylate was 1.41 mol%. Mole%, the random rate is 52%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| logarithmic viscosity | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com