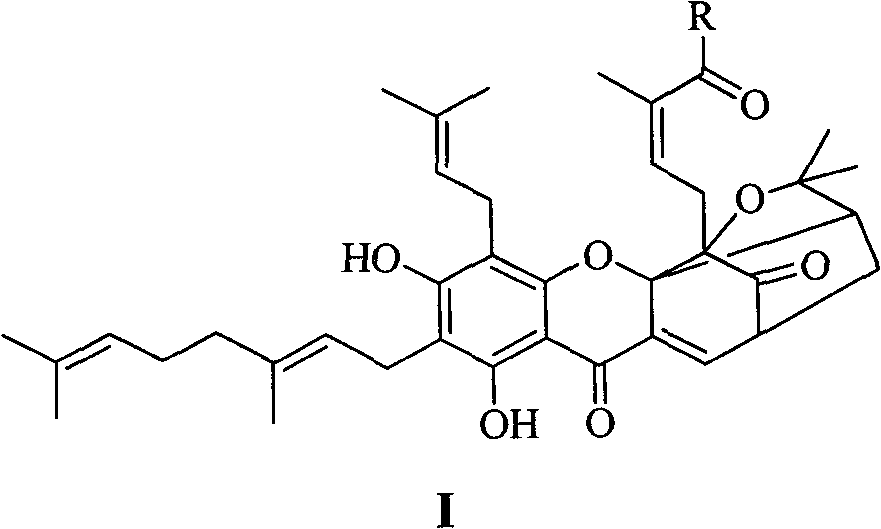
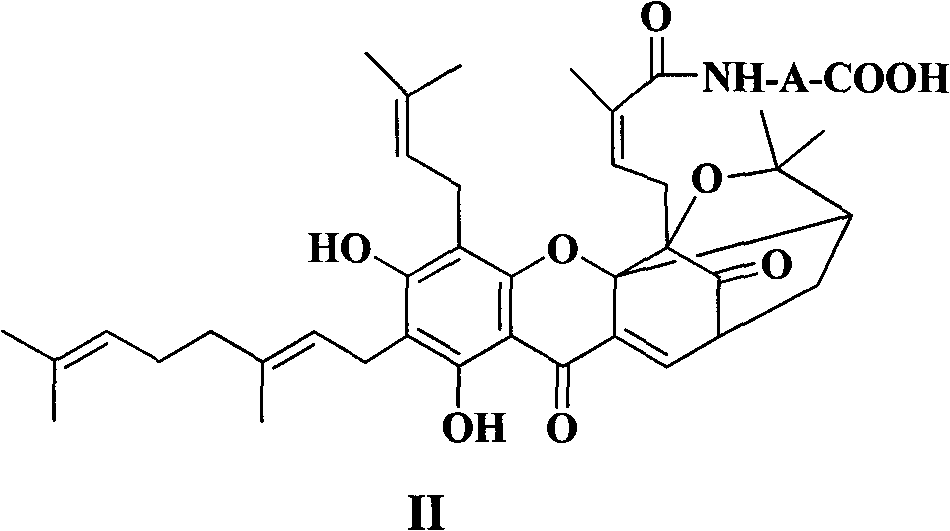
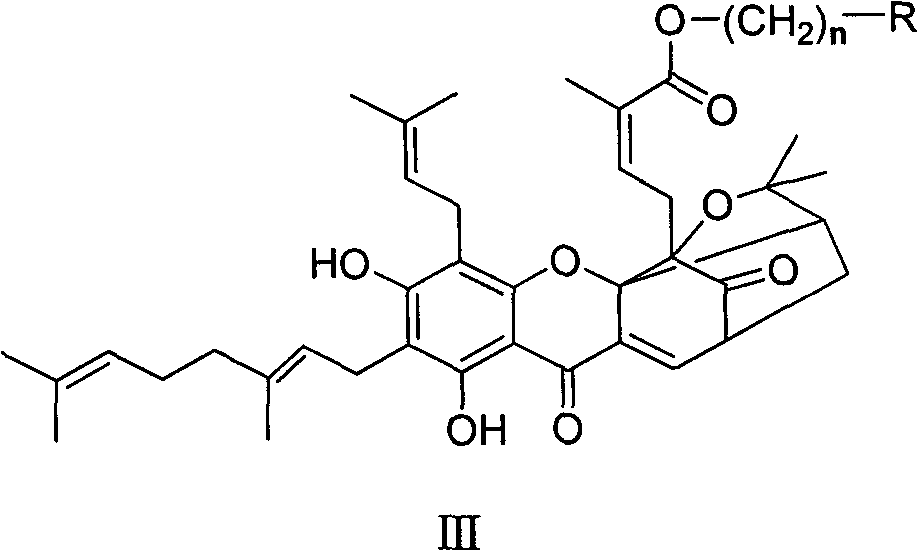
Neogambogic acid derivative, preparation method thereof and pharmaceutical application
A technology of new gambogic acid and derivatives, applied in the fields of medicinal chemistry and drug therapy, can solve the problems of low solubility of new gambogic acid and influence on medicinal value, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] N, N-dimethyl new gambogic amide (I 1 )Synthesis
[0162] In a dry 25 mL round bottom flask were added neogambogic acid (315 mg, 0.5 mmol), DMAP (6 mg, 0.5 mmol), dimethylamine (0.1 mL), dissolved in 10 mL of dichloromethane. EDCI (115 g, 0.6 mmol) was slowly added dropwise in an ice bath, and the reaction was stirred at room temperature after the drop was completed. The reaction progress was detected by TLC, and the reaction was complete after 24 h. Suction filtration, the filtrate was concentrated, and separated by silica gel column chromatography. Concentrate under reduced pressure and dry to obtain 256 mg of yellow wax. Yield: 78%. IR (KBr, cm -1 ): 1117, 1172, 1216, 1453, 1582, 1630, 1645, 1740, 2858, 2974, 3063, 3430. ESI-MS m / z: 658 [M+H] + ; 1 H NMR (CDCl 3 , 300Hz, δ): 12.86 (1H, s, 6-OH), 7.43 (1H, d, J=6.9Hz, H-10), 5.72 (1H, t, J=7.1Hz), 5.14 (1H, t , J=7.0Hz, H-3), 5.05(1H, m, H-37), 5.02(1H, m, H-32), 3.56(1H, s), 3.40(1H, t, J=4.6, 6.7Hz), 3.23-...
Embodiment 2
[0164] N, N-diethyl neogambogic amide (I 2 )Synthesis
[0165] In a dry 25 mL round bottom flask were added neogambogic acid (315 mg, 0.5 mmol), DMAP (6 mg, 0.5 mmol), diethylamine (0.1 mL), dissolved in 10 mL of dichloromethane. EDCI (115 g, 0.6 mmol) was slowly added dropwise in an ice bath, and the reaction was stirred at room temperature after the drop was completed. The reaction progress was detected by TLC, and the reaction was complete after 24 h. Suction filtration, the filtrate was concentrated, and separated by silica gel column chromatography. Concentrate under reduced pressure and dry to obtain 277mg of yellow wax. Yield: 81%. IR (KBr, cm -1 ): 1117, 1172, 1216, 1453, 1562, 1629, 1648, 1738, 2832, 2965, 3060, 3425. ESI-MS m / z: 686 [M+H] + ; 1 H NMR (CDCl 3 , 300Hz, δ): 12.85 (1H, s, 6-OH), 7.43 (1H, d, J=6.9Hz, H-10), 5.72 (1H, t, J=7.1Hz), 5.14 (1H, t , J=7.0Hz, H-3), 5.05(1H, m, H-37), 5.01(1H, m, H-32), 3.56(1H, s), 3.40(1H, t, J=4.6, 6.7Hz), 3.23-3.36...
Embodiment 3
[0167] Neogamboylglycine (II 1 )Synthesis
[0168] Add neogambogic acid (315mg, 0.5mmoL), glycine methyl ester hydrochloride (75mg, 0.6mmol), DMAP (6mg, 0.5mmol) into a dry 25mL round bottom flask, and dissolve in 10mL of dichloromethane. Slowly add EDCI (115 g, 0.6 mmol) dropwise under ice bath, remove the ice bath after the drop, continue to stir at room temperature, and detect the reaction progress by TLC. After the reaction was completed, it was filtered with suction, and the filtrate was concentrated and subjected to column chromatography to obtain an orange-yellow wax. Dissolve with acetone, add 4% NaOH solution dropwise until the pH of the reaction solution reaches 11, stir the reaction at room temperature, and detect by TLC. After the reaction was completed, 10% hydrochloric acid was added to adjust the pH to 7, concentrated under reduced pressure, the residue was added with appropriate amount of water, and extracted with EtOAc. The organic layer was dried over anh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com