Process and apparatus for conversion of silicon tetrachloride to trichlorosilane
A technology of silicon tetrachloride and chlorosilane, applied in silicon halide compounds, chemical instruments and methods, silicon hydride, etc., can solve the problem of low yield of trichlorosilane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3a
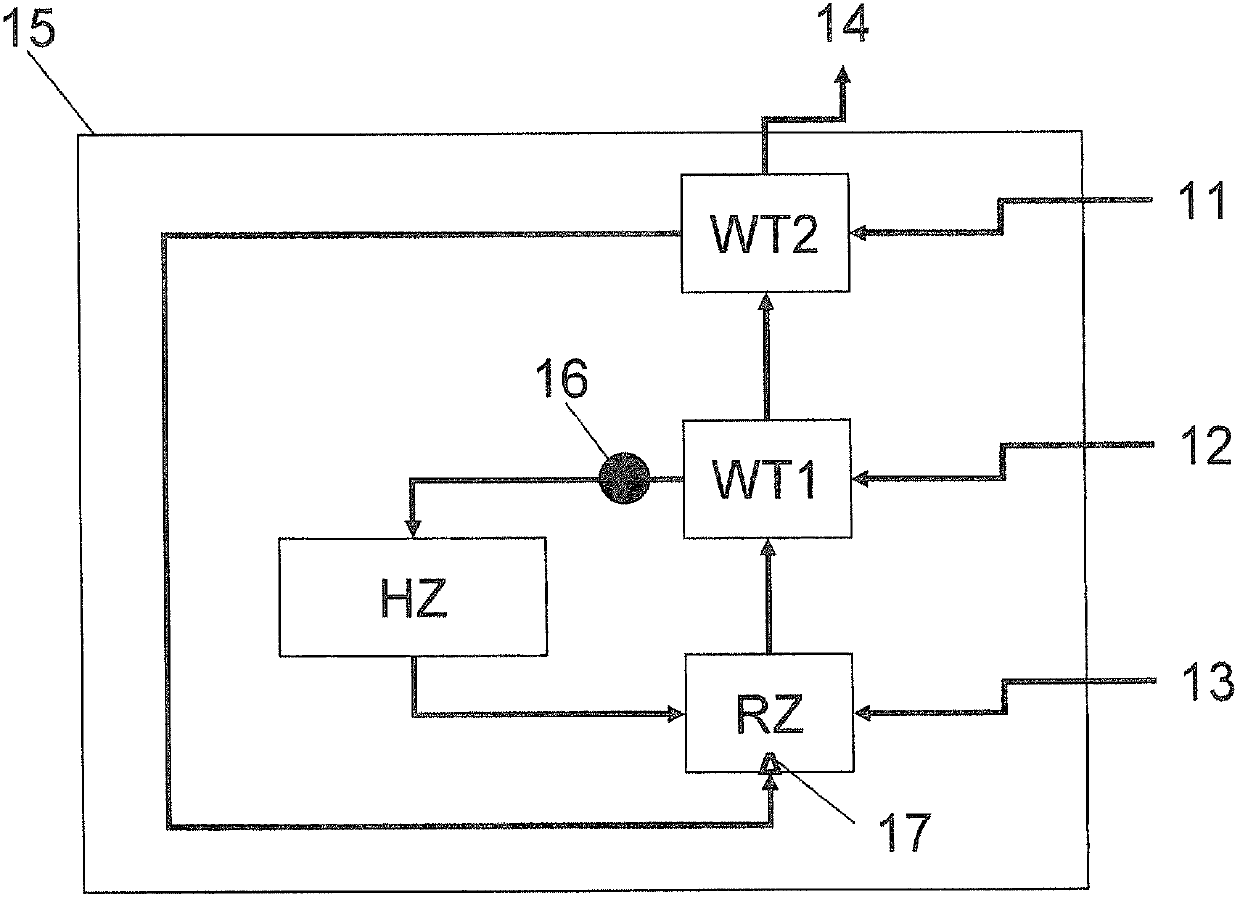
[0130] In this example, using the figure 1 of the reactor.
[0131] For the heat exchanger unit WT1, isobaric graphite was used.
[0132] According to the invention, the process was carried out similarly to Comparative Example 1, except that hydrogen and silicon tetrachloride were heated separately.
[0133] The hydrogen is raised to a temperature of 500°C in the second heat exchanger and introduced directly into the reaction zone before the hydrogen is mixed with the silicon tetrachloride in the reaction zone, and the tetrachloride is mixed with the silicon tetrachloride in the first heat exchanger. The silicon carbide is preheated to about 920°C and then heated to 1350°C by means of an electric heating element. The average temperature in the reaction zone is about 1000°C.
[0134] The relative selectivity of the reactor was increased to 145%.
[0135] It was also found that after shutting down the reactor, the heater was still intact. Any effect of methanation could not...
Embodiment 3b
[0137] Example 3b was carried out similarly to Example 3a, except that the pressure difference between the reactant side and the product side of the heat exchanger unit WT1 was in the range of 10 mbar to 1000 mbar by incorporating various throttling valves internal changes.
[0138] The optimum obtained has been found at a pressure difference between 50 mbar and 200 mbar while using a minimum graphite wall thickness in the range 4-30 mm (between reactant and product side of heat exchanger unit WT1). good result. However, optimum values are obtained with a minimum wall thickness between 10mm and 20mm.
[0139] The inventors have realized that the use of isobaric graphite for the heat exchanger unit WT1 in combination with a defined pressure difference seems to be optimal.
[0140] It appears that the low porosity of the material allows the formation of a diffuse volume flow to protect the graphite, however establishing a volume flow at a sufficiently low level does not reduce...
Embodiment 4
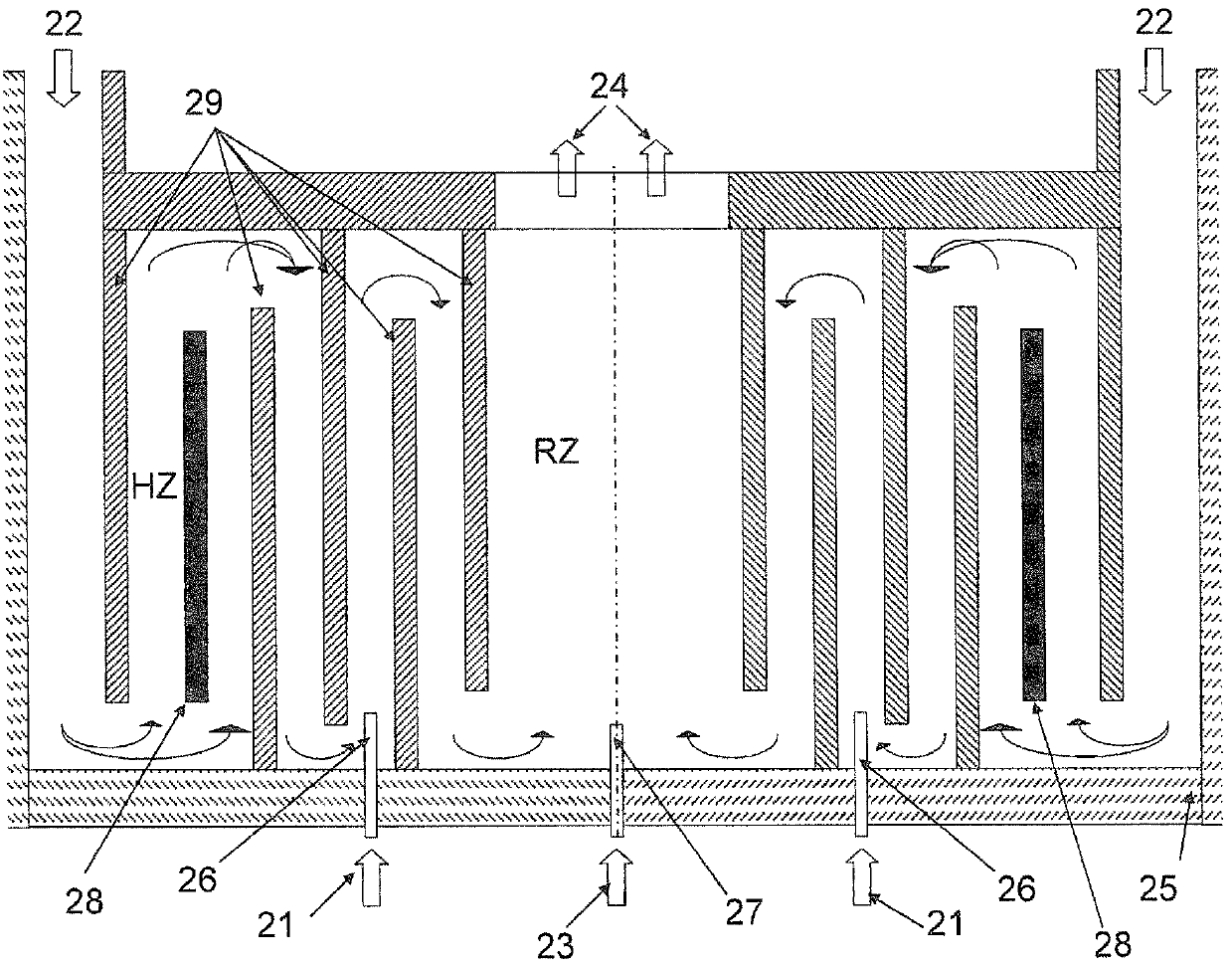
[0143] In addition to Example 3b, dichlorosilane was injected as a third reactant stream 13 in a molar ratio of 3% dichlorosilane to 97% silicon tetrachloride into an additional central nozzle installed at the bottom of the reaction zone. figure 1 A corresponding device with an additional central nozzle is schematically shown in .
[0144] The injected dichlorosilane stream has a temperature between 250°C and 350°C.
[0145] A temperature calibration to preheat the silicon tetrachloride stream in the heating zone is not necessary.
[0146] Use the center nozzle for the inflow of dichlorosilane.
[0147] The central nozzle for introducing dichlorosilane has a diameter of 15 mm.
[0148] This relative selectivity was further improved to 165% relative to structural problems without any apparent adverse effects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com