Camptothecin derivative and preparation method thereof as well as application of camptothecin derivative in preparation of medicine for treating tumours
A tumor drug, camptothecin technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of limiting efficacy and dosage, and achieve the effects of increasing water solubility, improving biological activity, and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
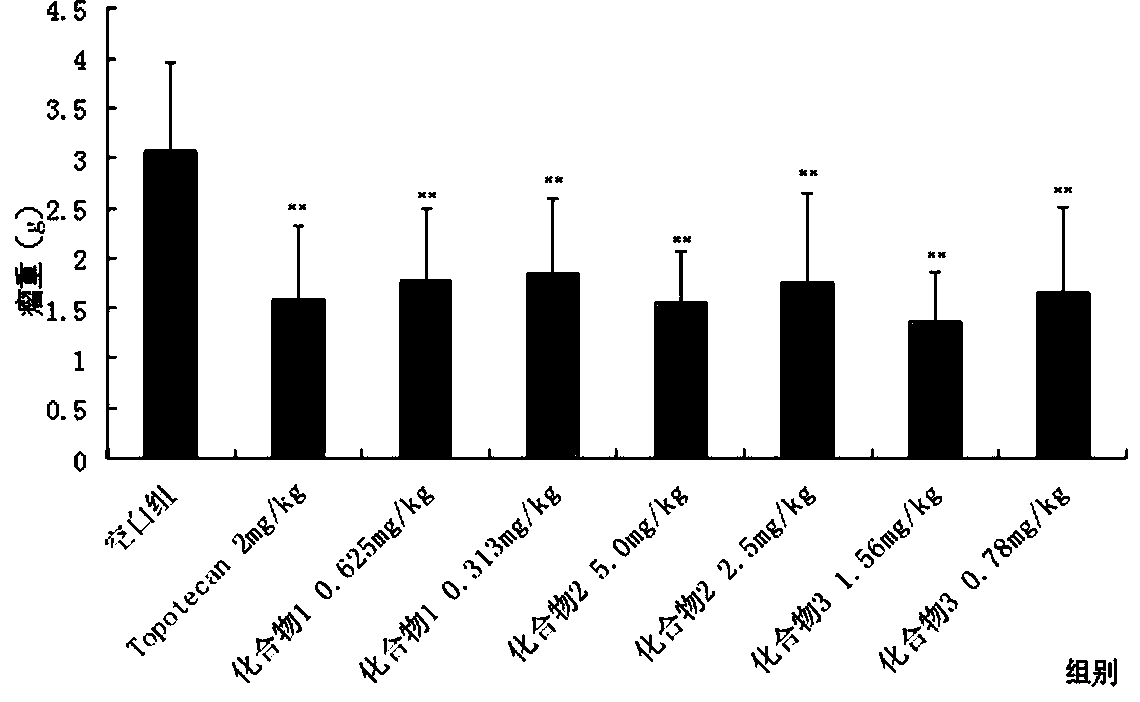
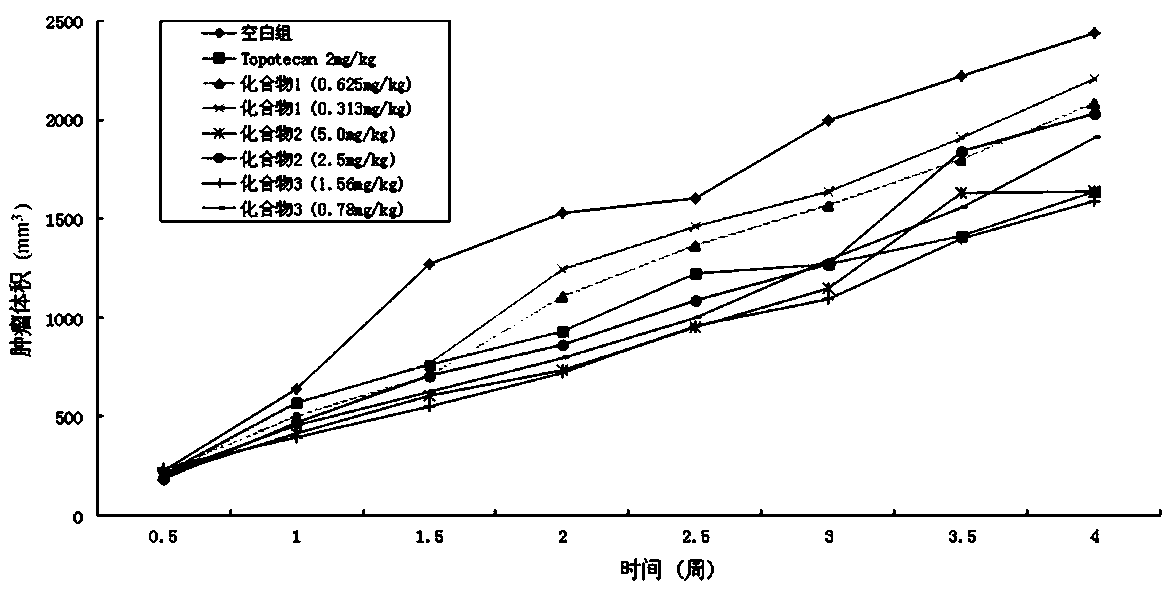
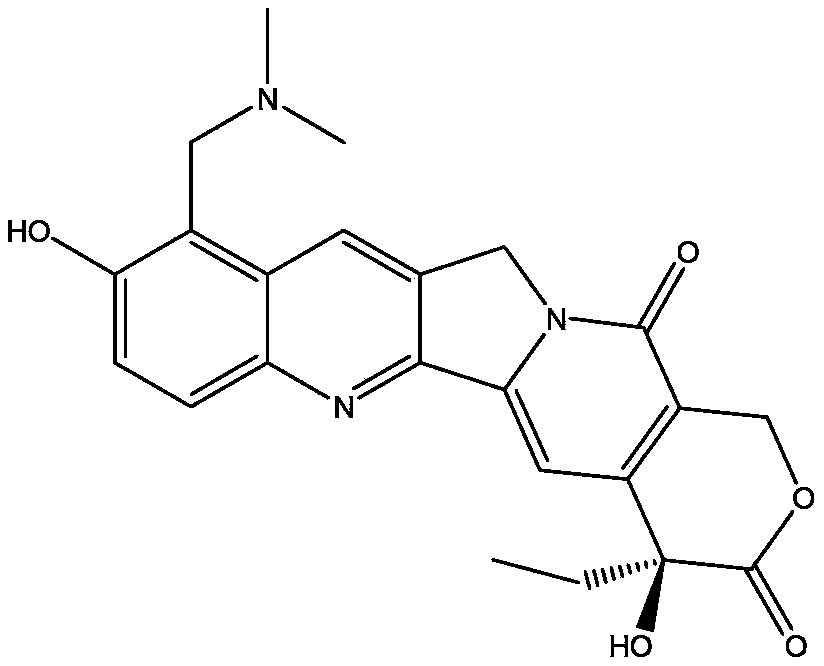
Image
Examples
Embodiment 1
[0070] A camptothecin derivative with a chemical name: 20-{[(2Z)-3-carboxy-2-acryloyl]amino}acetoxycamptothecin, whose chemical structural formula is as follows:
[0071]
[0072] The preparation method of above-mentioned camptothecin derivatives comprises the following steps, and its synthetic route is shown in image 3 :
[0073] Camptothecin (compound 60, 1.2g, 3.45mmol) aqueous solution, scandium trifluoromethanesulfonate (1.018g, 2.07mmol), dimethylaminopyridine (DMAP, 1.264g, 10.3mmol), 80mL of anhydrous dichloro After mixing methane (DCM), cool to -8°C in an ice-salt bath, add 1.8g (10.3mmol) 2-(tert-butoxy-carbonyl amido)-acetic acid after 30min, stir at -8°C for 30min, add 3.557g (17.2mmol) dicyclohexylcarbodiimide (DCCI), continue to stir at -8°C for 30min, then place at room temperature for 24h, filter the reaction mixture, and successively wash with 2×30mL 0.1N hydrochloric acid, 30mL distilled water, 30mL saturated NaCl Solution wash. The organic phase of th...
Embodiment 2
[0080] A camptothecin derivative, chemical name: 20-{[(2Z)-3-carboxy-2-acryloyl]amino}acetoxy-10-propanesulfonyloxy-9-(dimethylamino) Methylcamptothecin, its chemical structural formula is as follows:
[0081]
[0082] The preparation method of above-mentioned camptothecin derivatives comprises the following steps, and its synthetic route is shown in Figure 4 :
[0083] Topotecan (compound 80, 0.2g, 0.44mmol) and dimethylaminopyridine (0.04g, 0.33mmol) were mixed, then dissolved in 10mL DMF, the solution was cooled to 0°C, 0.62mL triethylamine was added, and then Add 5mL of n-propylsulfonyl chloride (0.40mL, 3.52mmol) aqueous solution dropwise, react under magnetic stirring at 0°C for 6h, add 20mL of water to the reaction mixture, extract with 20mL×3 times of dichloromethane, and dissolve with 10% hydrochloric acid The pH of the solution layer was adjusted to 7, and then the precipitated solid was filtered by suction to obtain 0.11g bright yellow compound 85 ( Figure 4...
Embodiment 3
[0091] A camptothecin derivative, chemical name: 20-{[(2Z)-3-carboxy-2-acryloyl]amino}acetoxy-10-isopropylsulfonyloxy-9-(dimethylamino ) methyl camptothecin, its chemical structural formula is as follows:
[0092]
[0093] The preparation method of above-mentioned camptothecin derivatives comprises the following steps, and its synthetic route is shown in Figure 5 :
[0094] Mix topotecan (compound 80, 0.2g, 0.44mmol) and dimethylaminopyridine (0.04g, 0.33mmol), then dissolve in 10mL DMF, cool the solution to 0°C, add 0.92mL triethylamine, and gradually Add 5mL of isopropylsulfonyl chloride (0.76mL, 6.60mmol) aqueous solution dropwise, react for 5h under magnetic stirring at 0°C, add 30mL of water to the reaction mixture, extract with 20mL×3 times of dichloromethane, and dissolve with 10% hydrochloric acid The pH of the solution layer was adjusted to 7, and then the precipitated solid was filtered with suction to obtain 0.14 g of bright yellow compound 105, with a yield o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com