A kind of berberine ultrafine intestinal adhesion type sustained-release pellets and preparation method thereof
A technology of slow-release pellets and berberine, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, block delivery, etc., can solve the problems of short residence time and low effective bioavailability, and achieve simple preparation methods, Improve the effective bioavailability and the effect of taking convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1. Preparation of berberine homogeneous suspension
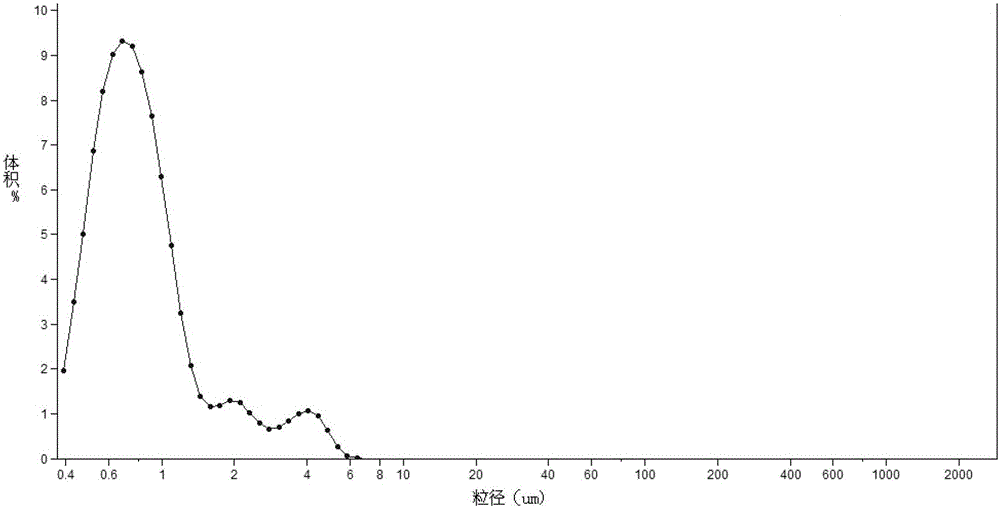
[0015] Prepare 2.5% (w / v) HPMC aqueous solution as the carrier during grinding, weigh 500g of 2.5% (w / v) HPMC aqueous solution and pour it into the grinding cup, turn on the mechanical stirring and grinding machine, and continuously add the pre-weighed 150g Berberine hydrochloride, until the berberine hydrochloride is all added to the grinding cup, start timing, the grinding speed is 3600rpm, after grinding for 1 hour, take out the ground material from the feeding port to obtain the berberine suspension, ready for use. The particle size measurement results of the suspension are shown in Table 1.
[0016] Table 1 Particle size measurement results of berberine suspension
[0017] Time (min)
Mean(μm)
SD(μm)
<1μm (%)
<10μm (%)
d. 90 .(μm)
60
1.034
0.844
72.5
100
1.904
[0018] 2. Preparation of blank core
[0019] Weigh 500g of microcrystalline cellulose ...
Embodiment 2
[0026] Stir and grind an appropriate amount of hydroxypropyl cellulose aqueous solution with berberine to prepare a small and uniform berberine drug suspension; take an appropriate amount of blank pellet cores prepared by centrifugal granulation or extrusion spheronization In the chemical bed, spray the berberine drug suspension to make berberine small particle size drug-loaded pellets, and screen the pellets with a particle size of 0.25-0.43mm; EudragitRL, EudragitRS, EudragitL100, castor oil and polyethylene glycol Alcohol-1500 is made into a coating liquid, and coated to obtain berberine ultrafine intestinal adhesion type sustained-release pellets.
Embodiment 3
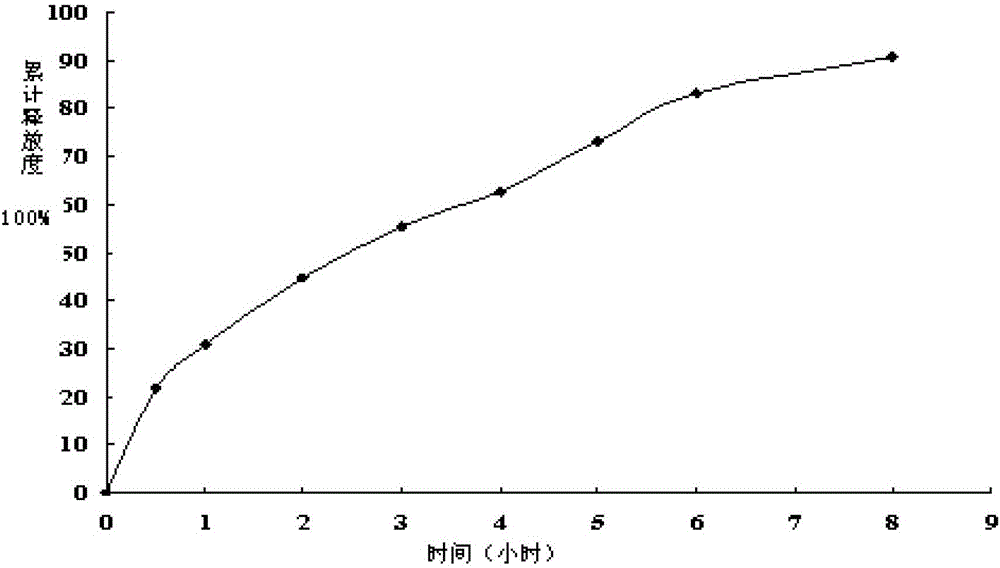
[0028] Pharmacokinetic fitting of berberine ultramicroparticle intestinal adhesion sustained-release pellets
[0029] Take the berberine ultrafine intestinal adhesion type sustained-release pellets prepared in Example 1, measure its release curve, and fit it with a commonly used release model. The fitting results are shown in Table 2:
[0030] Table 2 Pharmacokinetic fitting results of berberine ultrafine-grained intestinal-adhesive sustained-release pellets
[0031] model
[0032]From the fitting results, it can be seen that when the berberine ultrafine-grained intestinal-adhesive sustained-release pellets are fitted by the Higuchi equation, r>0.99, which is better than the other two models, and the fitting degree of the in vitro release model is as follows: Higuchi model > Zero-order model > First-order model. The three models in the above table are all proposed based on Fick’s diffusion law, and they obtain the approximate solution of Fick’s diffusion law on the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com