Berberine ultrafine-particle intestinal adhesion-type sustained-release pellet and preparation method thereof
A technology for sustained-release pellets and berberine, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and bulk delivery, etc., can solve the problems of low effective bioavailability and short residence time, and achieves improved effective bioavailability. degree, the preparation method is simple, and the effect of reducing the absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1. Preparation of berberine homogeneous suspension
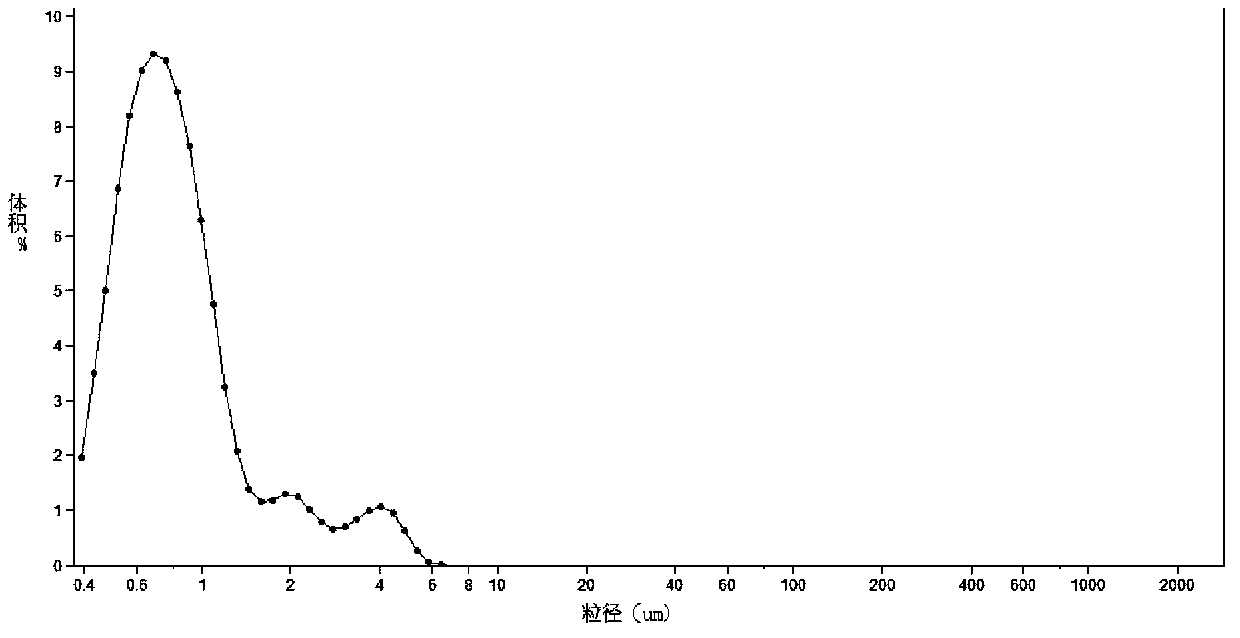
[0015] Prepare 2.5% (w / v) HPMC aqueous solution as the carrier during grinding, weigh out 500g of 2.5% (w / v) HPMC aqueous solution and pour into the grinding cup, turn on the mechanical stirring and grinder, and continuously add the pre-weighed 150g Berberine hydrochloride, until all the berberine hydrochloride is added to the grinding cup, start timing, the grinding speed is 3600 rpm, after grinding for 1 hour, the ground material is taken out from the receiving port to obtain the berberine suspension, which is ready for use. See Table 1 for the measurement results of the particle size of the suspension.
[0016] Table 1 Particle size measurement results of berberine suspension
[0017] Time (min)
Mean(μm)
SD(μm)
<1μm (%)
<10μm (%)
d. 90 .(μm)
60
1.034
0.844
72.5
100
1.904
[0018] 2. Preparation of blank pellet core
[0019] Weigh 500g of microcrystalline cellulose and place it in a centrifugal coating granulato...
Embodiment 2
[0026] Stir and grind an appropriate amount of hydroxypropyl cellulose aqueous solution with berberine to prepare a small and uniform berberine drug suspension; take an appropriate amount of blank pellets prepared by centrifugal granulation or extrusion spheronization starch to flow In the fluidized bed, spray the berberine drug suspension to make berberine small particle size drug-loaded pellets, and screen the pellets with a particle size of 0.25-0.43mm; Eudragit RL, Eudragit RS, Eudragit L100, castor oil and Polyethylene glycol-1500 is made into a coating solution and coated to obtain berberine ultrafine intestinal adhesion type sustained-release pellets.
Embodiment 3
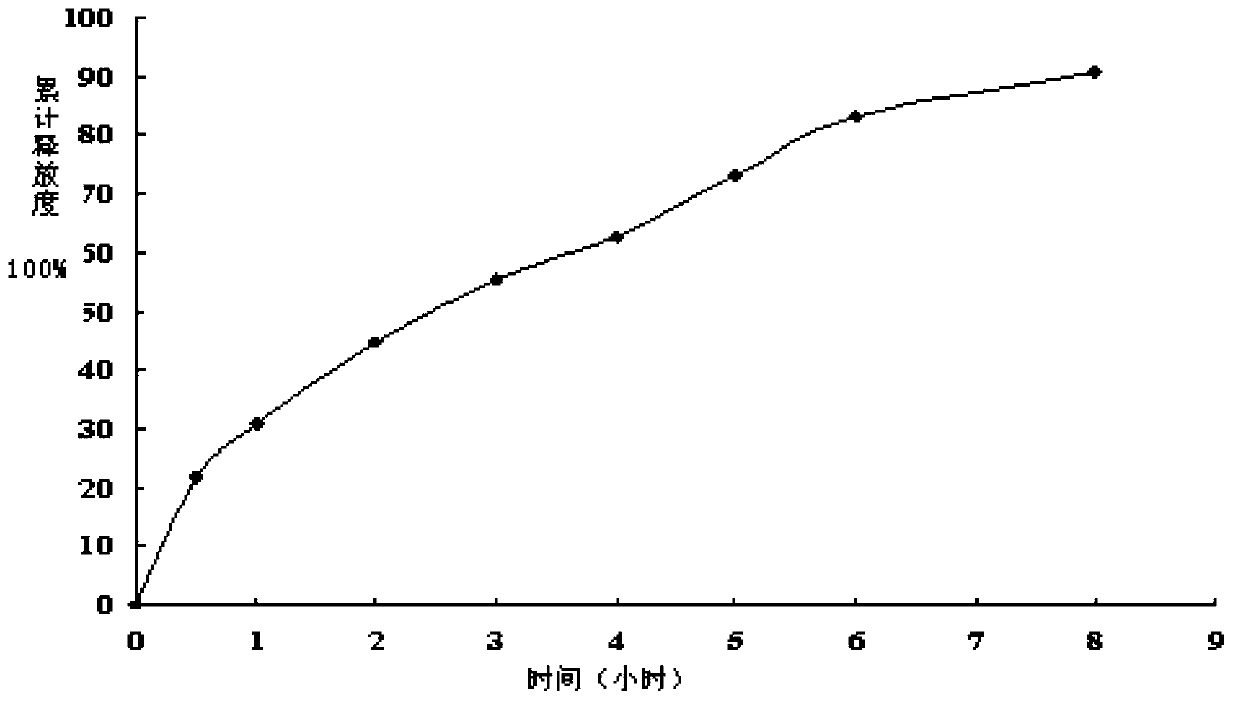
[0028] Pharmacokinetics fitting of berberine ultrafine intestinal adhesion sustained-release pellets
[0029] Take the berberine ultrafine intestinal adhesion sustained-release pellets prepared in Example 1, measure the release curve, and fit it with the commonly used release model. The fitting results are shown in Table 2:
[0030] Table 2 pharmacokinetics fitting results of berberine ultrafine intestinal adhesion sustained-release pellets
[0031] Model
[0032] It can be seen from the fitting results that when the berberine ultrafine intestinal adhesion sustained-release pellets are fitted with Higuchi equation, r> 0.99, better than the other two models, and the closeness of the in vitro release model is as follows: Higuchi model> Zero-order model> First-order model. The three models in the above table are all based on Fick’s diffusion law. They are based on some boundary conditions and assumptions to obtain approximate solutions to Fick’s diffusion law. Therefore, the drug in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com