Compound containing tetraphenyl ethylene unit, preparation method and applications thereof
A technology of tetraphenylethylene and compounds, which is applied in the field of optoelectronic devices, can solve problems such as unfavorable carrier transport, spectral red shift, and increased conjugate length, and achieve enhanced hole transport capabilities, mild reaction conditions, and improved device efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
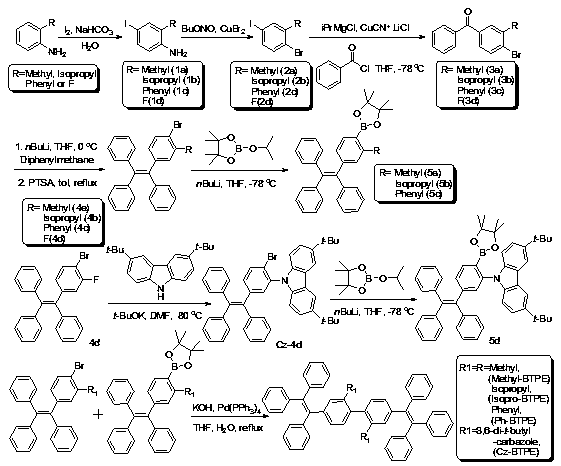
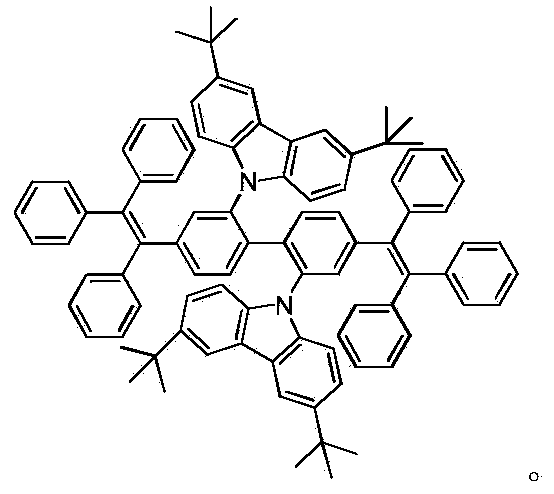
[0022] The synthesis of embodiment 1 compound Methyl-BTPE, Isopro-BTPE, Ph-BTPE and Cz-BTPE
[0023] (1) Arylamine (R is Or F) (60mmol), sodium bicarbonate (7.56g, 90mmol) and 150mL water were added in a 250mL round bottom flask and stirred vigorously, then the ground powdery elemental iodine (12.7g, 50mmol) was added in several times and ensured The addition was completed within half an hour, and the resulting mixture was continued to react at room temperature for one hour until the color of the iodine element disappeared completely. The reaction solution was suction filtered to obtain crude products 1a-1d, which were directly used in the next reaction. The general structural formula of the compound 1a-1d is where R is followed by H 3 C-(1a), (1b), (1c) and (1d);
[0024] (2) Dissolve compound 1a-1d (30mmol) and copper bromide (8.38g, 37.5mmol) in 150mL acetonitrile solution, then add tert-butyl nitrite (3.87g, 37.5mmol) dropwise to the reaction system . After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com