A kind of monoazo dye and its synthesis method and application
A technology for monoazo and dyes, applied in the direction of monoazo dyes, azo dyes, organic dyes, etc., can solve the problem of reducing the solubility of organic dyes, reducing the area of full-color gamut, and increasing the degree of dye conjugation, etc. problems, to achieve the effect of small rigidity, large color gamut area, and improved light stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Synthesis of Dye A
[0065]
[0066] Weigh 6.6g (132g / mol, 0.05mol) of tert-butoxycarbonylhydrazine and dissolve it in 50mL of n-hexane, weigh 6.7g (114g / mol, 0.058mol) of isooctylaldehyde and slowly add it dropwise to the above solution, heat up to reflux reaction 2h. After the reaction was completed, the solvent was evaporated to dryness to obtain compound 11 with a yield of 100%. Cool compound 11 to 0-5°C, weigh 100 mL of 1 mol / L borane tetrahydrofuran solution and slowly add it dropwise into the reaction device. The developer is dichloromethane / petroleum ether=1:1, and react for 2 hours to obtain compound 12.
[0067] Measure 15mL of concentrated hydrochloric acid, slowly add it dropwise to the above solution, heat to reflux after no obvious bubbles, and react for 0.5h. After the reaction was completed, it was cooled, and the precipitated boric acid was removed by suction filtration, and the solution was rotary evaporated to dryness. Compound 13 was obtained w...
Embodiment 2
[0078] Synthesis of Dye B
[0079]
[0080] Weigh 1.29g (129g / mol, 0.01mol) of 2,6-difluoroaniline, dissolve it in 9ml of concentrated hydrochloric acid and 50ml of water, cool to 0-5°C, weigh 0.8g (1.2mol, 69g / mol) of sodium nitrite Dissolve it in 5ml of water, add diazotization for 2 hours at one time, and obtain compound 16. Weigh 2.52g (252g / mol, 0.01mol) of compound 14, dissolve it in 50ml of ethanol, cool to 0-5°C, slowly add diazonium salt 16 dropwise to the above solution, and use aqueous sodium hydroxide solution to control the pH of the solution to 9. After the coupling is completed, filter and dry to obtain dye B.
[0081] After column chromatographic separation, the NMR spectrum is characterized, and the NMR spectrum data is: 1 H NMR (CDCl 3 ): 14.23(s, 1H), 7.224(d, 1H), 6.722(d, 2H), 3.627(m, 2H), 2.553(t, 2H), 1.652(m, 2H), 1.552(m, 1H) , 1.338 (m, 10H), 0.904 (m, 9H).
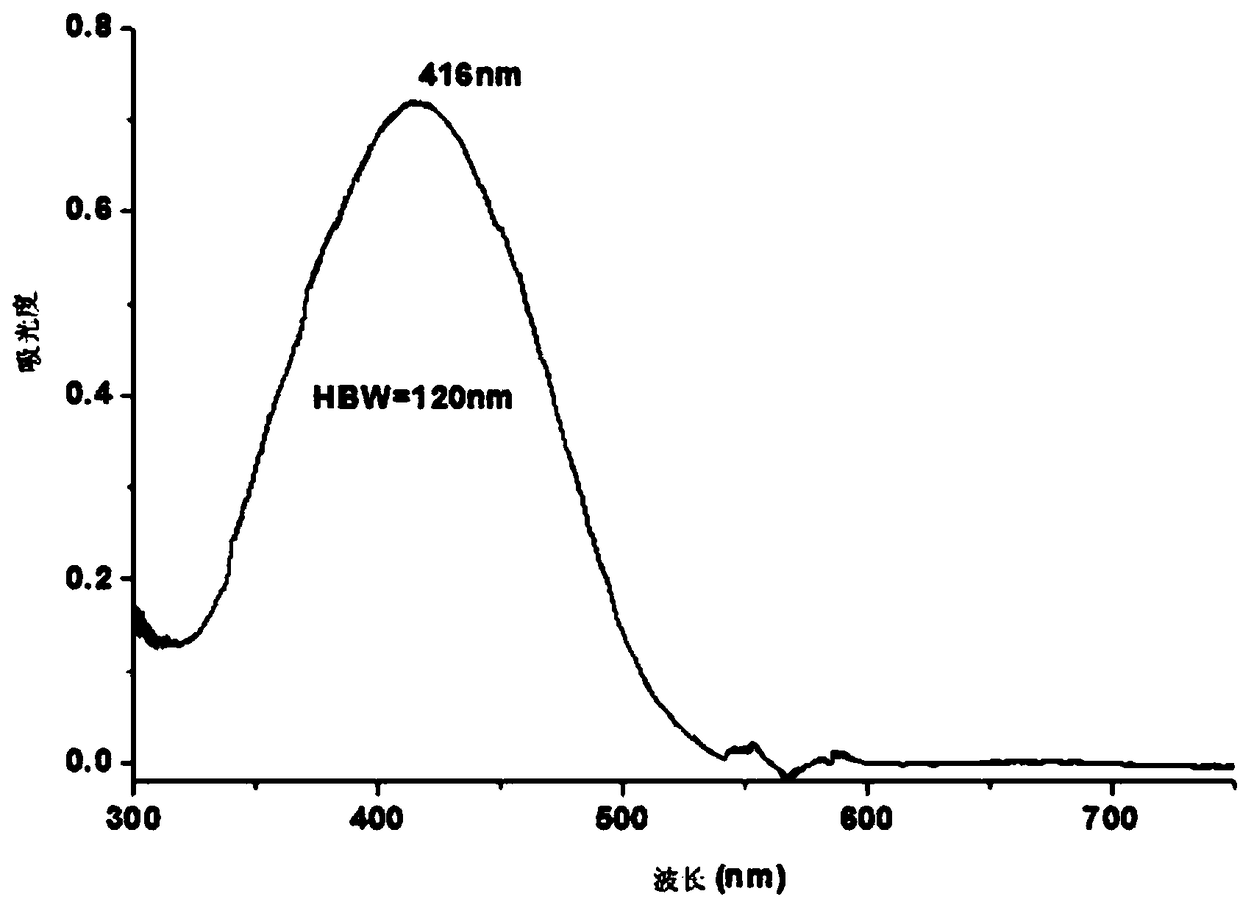
[0082] The UV-Vis absorption spectrum of dye B is as follows Figure 8 As shown in...
Embodiment 3
[0085] According to the similar synthetic method of Examples 1-2, compound C was prepared using different substrates, and its synthetic route and structural formula are as follows:
[0086]
[0087] Purified by column chromatography and then characterized by proton nuclear magnetic spectrum, the data of proton nuclear magnetic spectrum are 1 H NMR (CDCl 3 ): 14.22(s, 1H), 7.444(d, 2H), 7.512(d, 2H), 3.657(m, 2H), 2.453(t, 2H), 1.662(m, 2H), 1.562(m, 1H) , 1.348(m, 10H), 0.914(m, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com