1,ω-bis(3,6-diarylcarbazolyl)-alkane and its preparation method
A technology of diarylcarbazolyl and naphthylcarbazolyl is applied in the field of 1,ω-bis-alkane and its preparation, and can solve the problems of reducing the external quantum efficiency to only
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
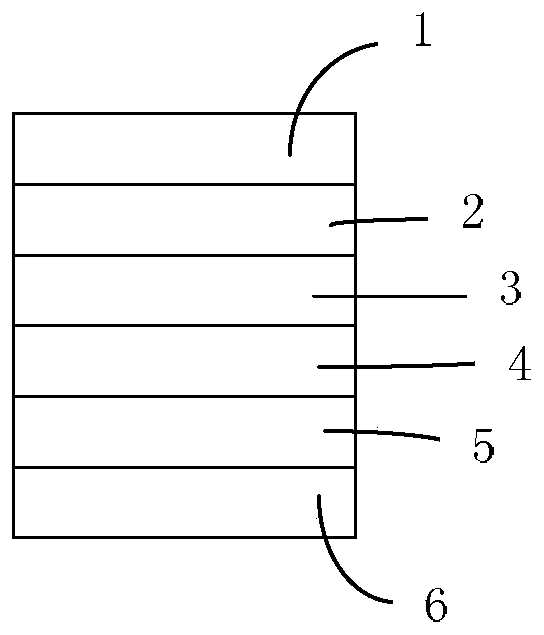
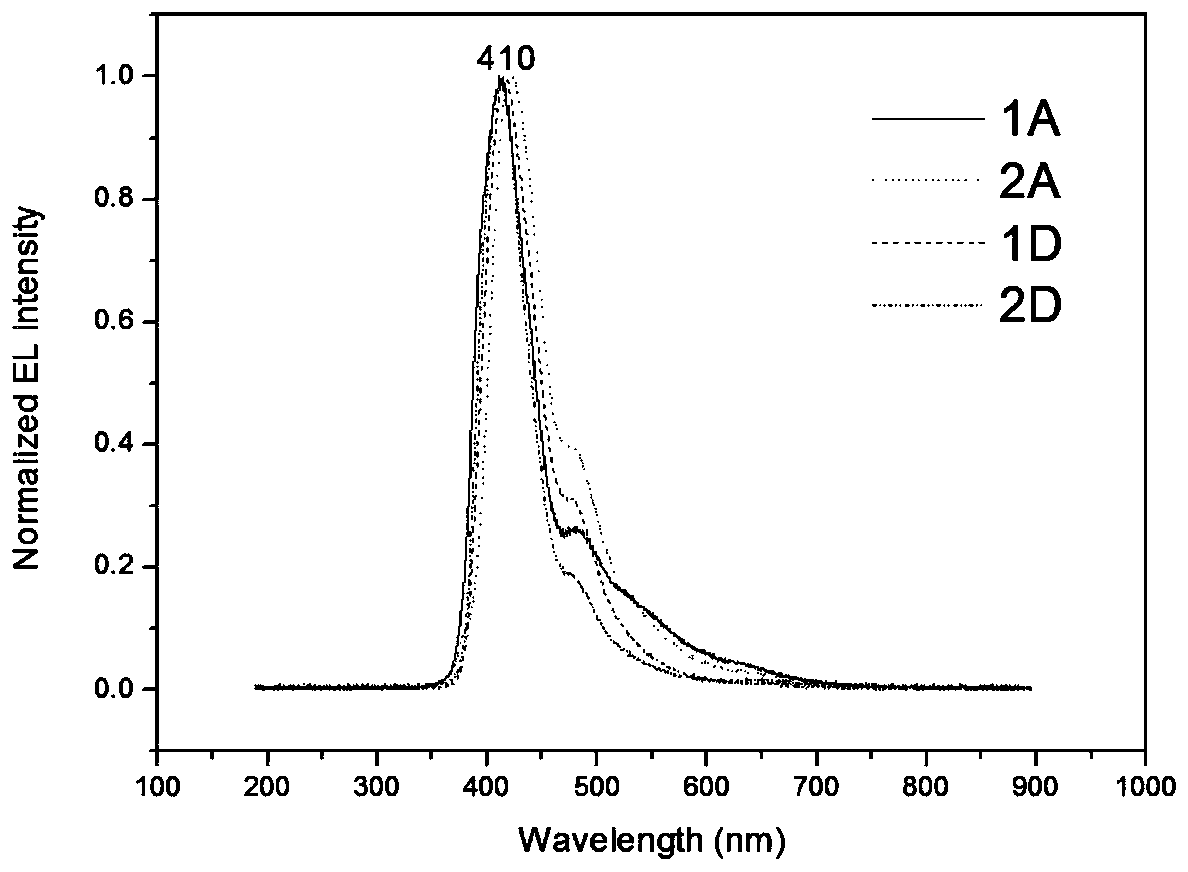
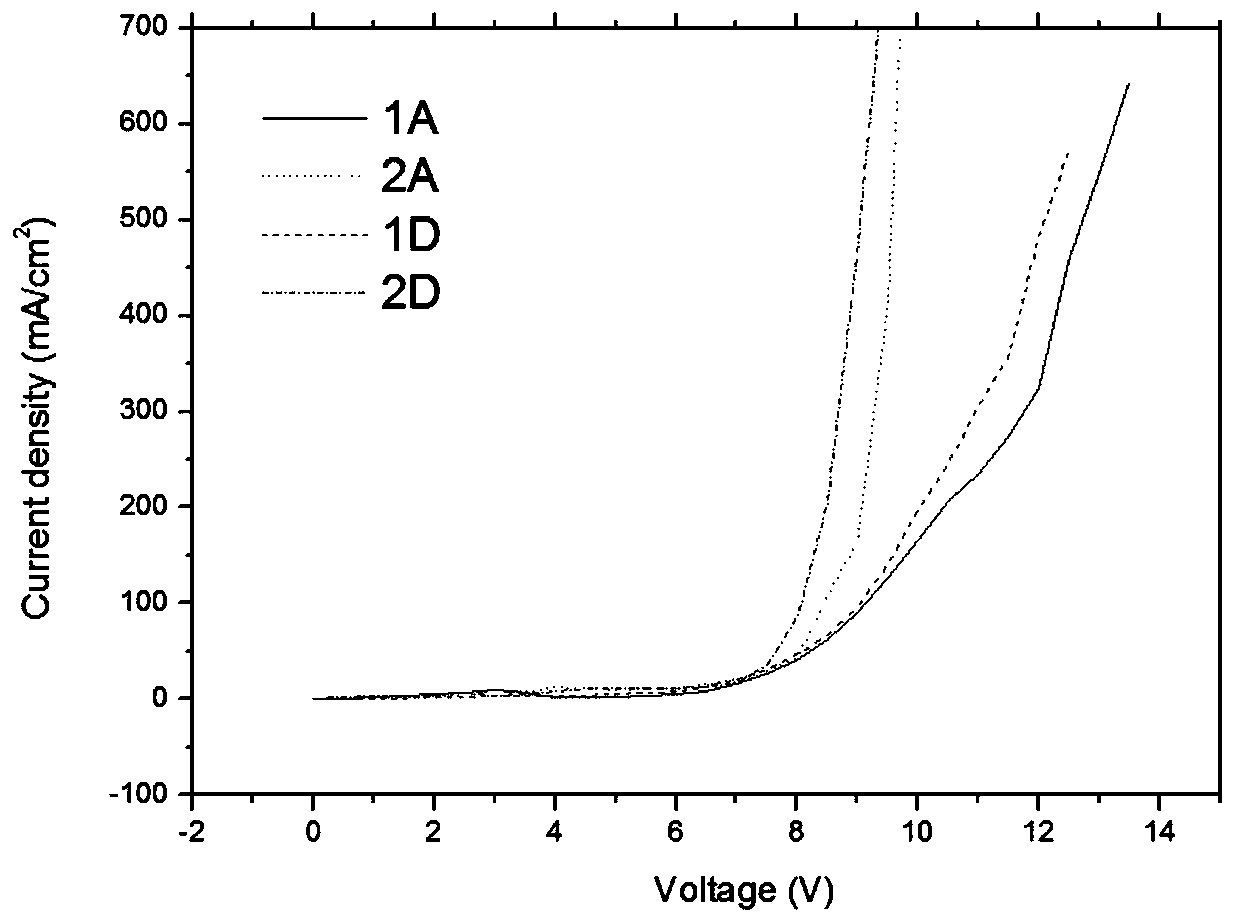
Image
Examples
Embodiment 1
[0031] (Ⅰ) Synthesis of 3,6-diphenyl-9H-carbazole, the reaction formula is as follows:
[0032]
[0033] Add 3,6-dibromo-9H-carbazole (2.6g, 8mmol), NaBPh4 (2.736g, 8mmol), tetrabutylammonium bromide (TBAB) (0.12g, 0.5mmol), chlorinated into a 250mL three-necked flask Palladium (0.05g, 0.25mmol), 2M sodium carbonate solution 30ml, acetone 120ml, refluxed under nitrogen atmosphere for 36h. The acetone was evaporated, the aqueous layer was extracted with dichloromethane, the extract was washed with water and dried over anhydrous magnesium sulfate, and separated by silica gel column chromatography to obtain a white flocculent product 3,6-diphenyl-9H-carbazole (2.3g, yields: 89%), the eluent is petroleum ether: methylene chloride = 2:1. Melting point: 177.1°C.
[0034] The test results of 3,6-diphenyl-9H-carbazole are as follows:
[0035] Hydrogen spectrum:
[0036] 1 H NMR(CDCl 3 ,400MHz),δ(ppm):8.34(s,2H),7.73(d,J=7.6Hz,4H),7.71-7.68(m,2H),7.49(t,J=7.5Hz,6H),7.36 (t, J=7.4 Hz, 2H). ...
Embodiment 2
[0057] (Ⅰ) Synthesis of 3,6-diphenylmethyl-9H-carbazole, the reaction formula is as follows:
[0058]
[0059] Add 3,6-dibromo-9H-carbazole (2.6g, 8mmol), 4-methylphenylboronic acid (2.72g, 18mmol), tetrabutylammonium bromide (TBAB) (0.12g, 0.5 mmol), palladium chloride (0.05g, 0.25mmol), 30ml of 2M sodium carbonate solution, 120ml of acetone, reflux for 36h under nitrogen. The acetone was evaporated, the aqueous layer was extracted with dichloromethane, the extract was washed with water and dried with anhydrous magnesium sulfate, and separated by silica gel column chromatography to obtain a white flocculent product 3,6-benzyl-9H-carbazole (2.3g , Yields: 90%), the eluent is petroleum ether: methylene chloride = 2:1. Melting point: 204.0°C.
[0060] The test results of 3,6-Diphenylmethyl-9H-carbazole are as follows:
[0061] Hydrogen spectrum:
[0062] 1 H NMR(CDCl 3 ,400MHz),δ(ppm):8.46(s,2H),7.82-7.76(m,6H),7.57(s,2H),7.43(d,J=7.9Hz,4H),2.58(s,6H) .
[0063] Carbon spectrum:
[00...
Embodiment 3
[0083] (Ⅰ) Synthesis of 3,6-Diαnaphthyl-9H-carbazole
[0084]
[0085] Add 3,6-dibromo-9H-carbazole (2.6g, 8mmol), α-naphthylboronic acid (3.096g, 18mmol), tetrabutylammonium bromide (TBAB) (0.12g, 0.5mmol) into a 250mL three-necked flask ), palladium chloride (0.05g, 0.25mmol), 2M sodium carbonate solution 30ml, acetone 120ml, reflux for 36h under nitrogen atmosphere. The acetone was evaporated, and the aqueous layer was extracted with dichloromethane. The extract was washed with water and dried with anhydrous magnesium sulfate. The white flocculent product 3,6-diαnaphthyl-9H-carbazole (3.0g) was separated by silica gel column chromatography. , Yields: 90%), the eluent is petroleum ether: methylene chloride = 2:1. Melting point: 139.4°C.
[0086] The test results of 3,6-diαnaphthyl-9H-carbazole are as follows:
[0087] Hydrogen spectrum:
[0088] 1 H NMR(CDCl 3 ,400MHz),δ(ppm):8.23(s,1H),8.19(s,2H),7.99(d,J=8.5Hz,2H),7.91(d,J=8.0Hz,2H),7.87-7.85 (m, 2H), 7.58 (s, 4H), 7.56-7.48 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com