Method of treating neurological disorders with extracts of oleander spp. or oleander spp.
An extract, technology of oleander leaves, applied in the field of preparation, treatment of neurological diseases or disorders, can solve the problem of no dose-related toxicity found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 8
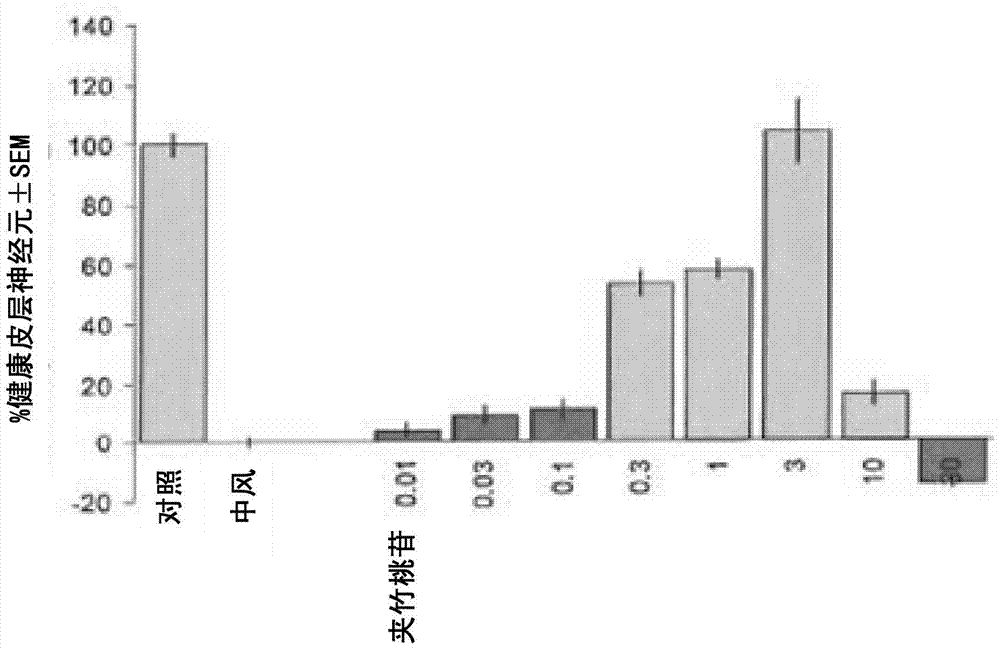
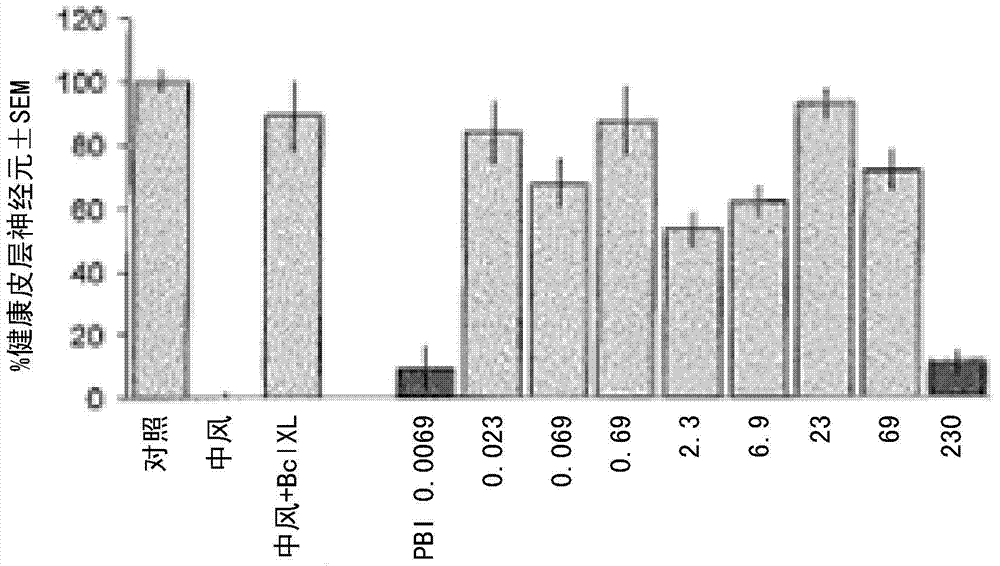
[0069] Example 8 details the in vitro assay used to evaluate the efficacy of the extract, or a composition thereof, in the treatment of stroke-induced ischemic nerve damage. The assay is a brain slice-based assay for oxygenated glucose deprivation (OGD), which is used to induce >50% loss of healthy cortical neurons within 24 hours. A total unfractionated SCF extract of Nerium species such as Nerium oleander was used as a positive control. This total extract was then fractionated according to Example 13 to provide fractions of the extract of Nerium species. Fractions were analyzed according to Examples 6, 14 and 17.
[0070] The HNMR of triterpenes is characterized by 7 methyl signals at upfield, an alkene proton at about 5.3 ppm and an oxidized methine signal at about 3.4 ppm, and an upfield (about 1.0–2.5 ppm) signal Many methylene and methine proton signals for . The HNMR spectrum ( Figures 9B-9I ) indicates that the main components are steroids and triterpenes. No sig...
Embodiment 9
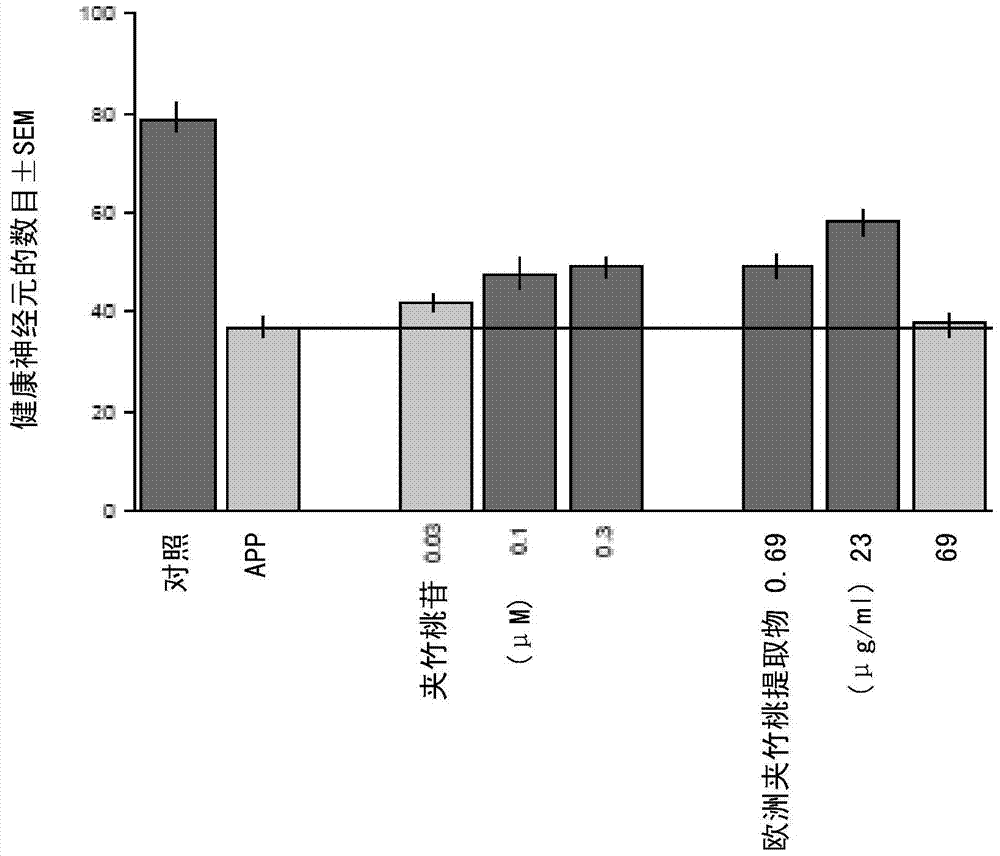
[0075] Example 9 details the in vitro assay used to evaluate the efficacy of the extract in treating Alzheimer's disease. This assay is a brain slice-based assay for APP / Aβ-induced (APP: β-amyloid precursor protein) degeneration of cortical pyramidal neurons. After being cleaved by secretase, APP is broken down into the Aβ peptide, which is believed to be the causative factor in the formation of β-amyloid plaques. The Aβ protein is involved in the formation of β-amyloid plaques and is believed to be the hallmark, if not the cause, of Alzheimer's disease. Biolistic transfection was used to introduce necessary markers such as YFP (labeled yellow fluorescent protein) and disease gene constructs into the same neuronal population of the brain slice. Co-transfection of YFP with APP isoforms resulted in progressive degeneration of cortical pyramidal neurons over a period of three to four days after brain slice preparation and transfection. data( Figures 2A-2C ) showed that oleand...
Embodiment 10
[0079]Example 10 details the assay used to evaluate the efficacy of the extract in the treatment of Huntington's disease. Mutant htt proteins were introduced into high-density, mixed co-cultures of cortical neurons, striatal neurons, and glia by electroporation. Striatal neurons and cortical neurons were transfected with fluorescent proteins of different colors, thereby facilitating the separate identification of different types of neurons in co-cultures. Color fluorescent proteins are fluorescent and "emit" color upon excitation with a light source of the appropriate wavelength. data( Figure 3A-3D ) showed that oleandrin and SCF extract of oleander were more effective than KW6002 (adenosine 2a receptor antagonist) in providing a greater number of surviving neurons. The data also indicate that the SCF extract is more effective than oleandrin alone, suggesting that the extract further comprises one or more therapeutically effective agents other than oleandrin, which could be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com