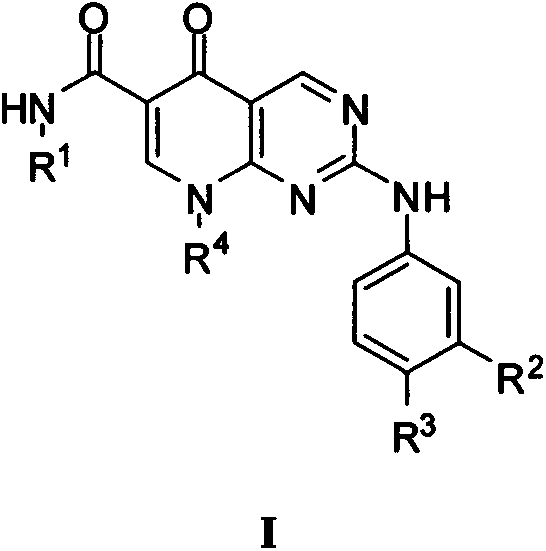
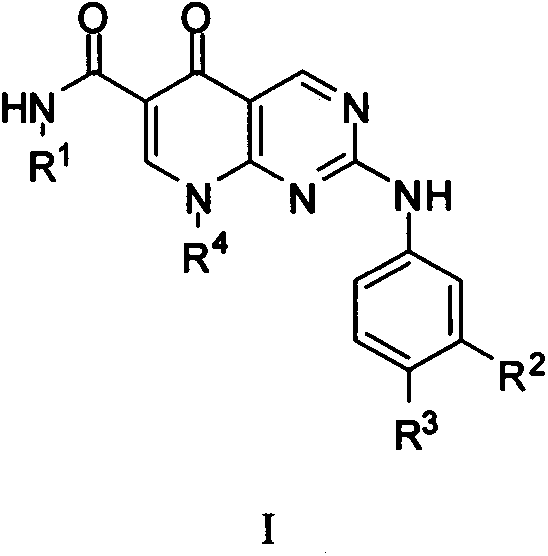
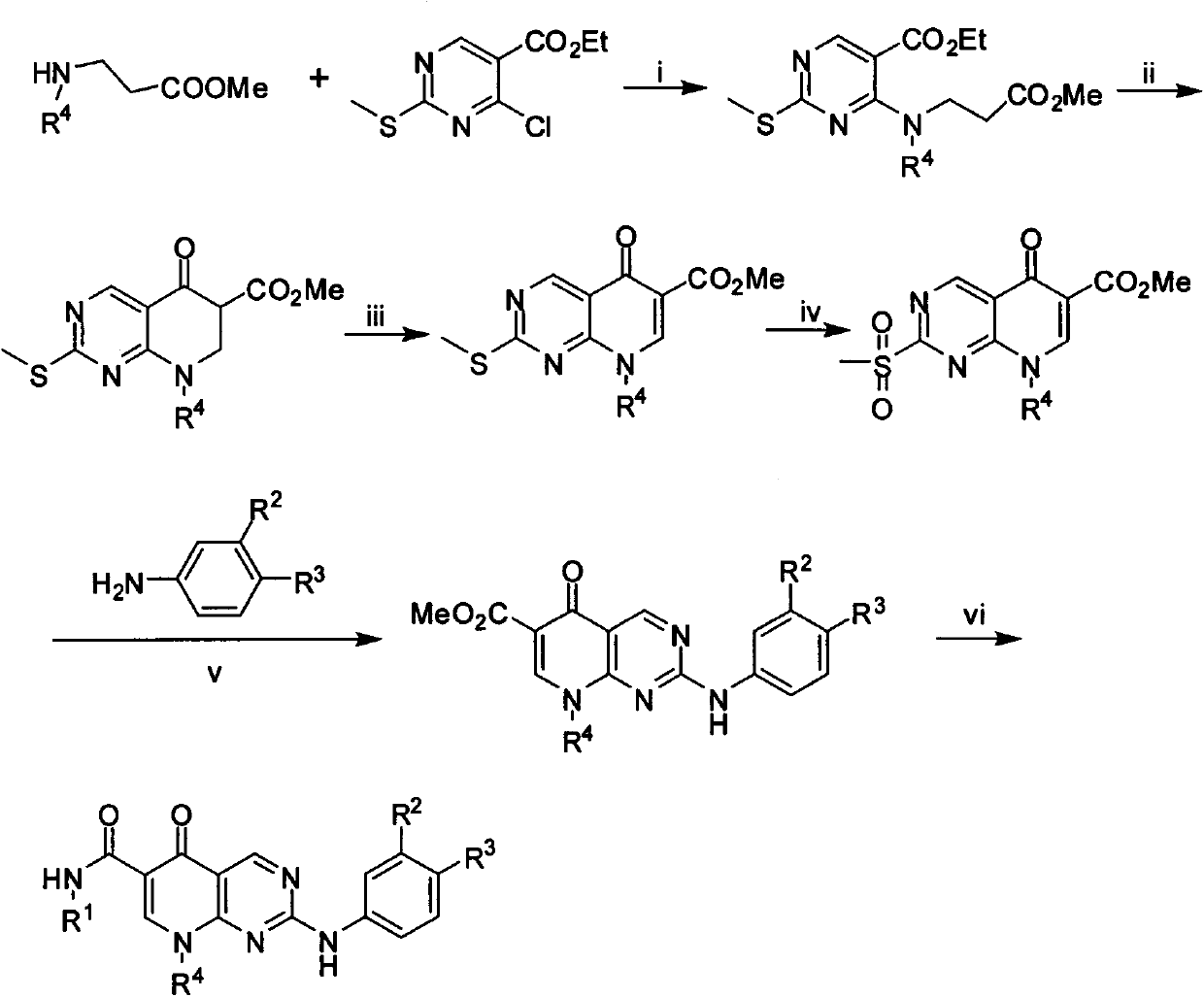
Pyridino-miazines PLK1 (Polo-like kinase 1) inhibitor and application thereof
A technology of pyrimidine and dihydropyridine, applied in the field of medicinal chemistry, can solve the problems of apoptosis, formation of tumor cell bipolar spindle, growth inhibition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 4-[isopropyl(3-methoxy-3-oxopropyl)amino]-2-methylthiopyrimidine-5-carboxylic acid ethyl ester (M1)
[0070] Add 0.579g of the compound 3-methyl isopropylaminopropionate, 5 drops of DIPEA, and 12ml of n-butanol into a 25ml medium reaction flask, stir well and cool in an ice bath for 5min, slowly add 2-methyl-6-chloropyrimidine- 0.928 g of ethyl 5-formate, stirred in an ice bath for 10 min, and reacted at room temperature for 9 h. Evaporate the solvent under reduced pressure, add 10ml of water to the residue, extract with ethyl acetate (10ml×3), dry the organic phase with anhydrous magnesium sulfate, concentrate and column chromatography (PE:EA=30:1) to obtain a white solid 0.9g, yield 66.1%, MS [M+H] + 342.1.
Embodiment 2
[0072] 4-[Cyclopentyl(3-methoxy-3-oxopropyl)amino]-2-methylthiopyrimidine-5-carboxylic acid ethyl ester (M2)
[0073] The preparation method was similar to M1, and 1.2 g of the sample was obtained, with a yield of 61.5%, MS [M+H] + 368.2.
Embodiment 3
[0075] Methyl 8-isopropyl-2-methylthio-5-oxo-5,6,7,8-tetrahydropyridin[2,3-d]pyrimidine-6-carboxylate (M3)
[0076] Add 10.36g of compound M, 3ml of toluene, and a small amount of t-BuOK into a 25ml reaction bottle, and the reaction turns yellow immediately. After 2h, add 20ml of ice water, extract with DCM (15ml×3), evaporate the solvent under reduced pressure, and concentrate the column layer Analysis gave 0.14g of yellow crystals, yield 44.9%, MS[M+H] +296.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com