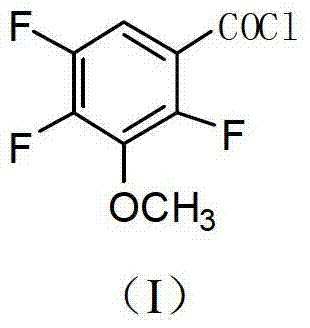
Industrial preparation method of 2,4,5-trifluoro-3-methoxybenzoyl chloride
A technology of methoxybenzoyl chloride and methoxybenzoic acid, which is applied in the field of industrial preparation of 2,4,5-trifluoro-3-methoxybenzoyl chloride, can solve the problem of high boiling point of aniline and insufficient substitution of fluorine. Thoroughness, high cost and other issues to achieve the effect of avoiding material loss, controlling reaction conversion rate, and avoiding environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
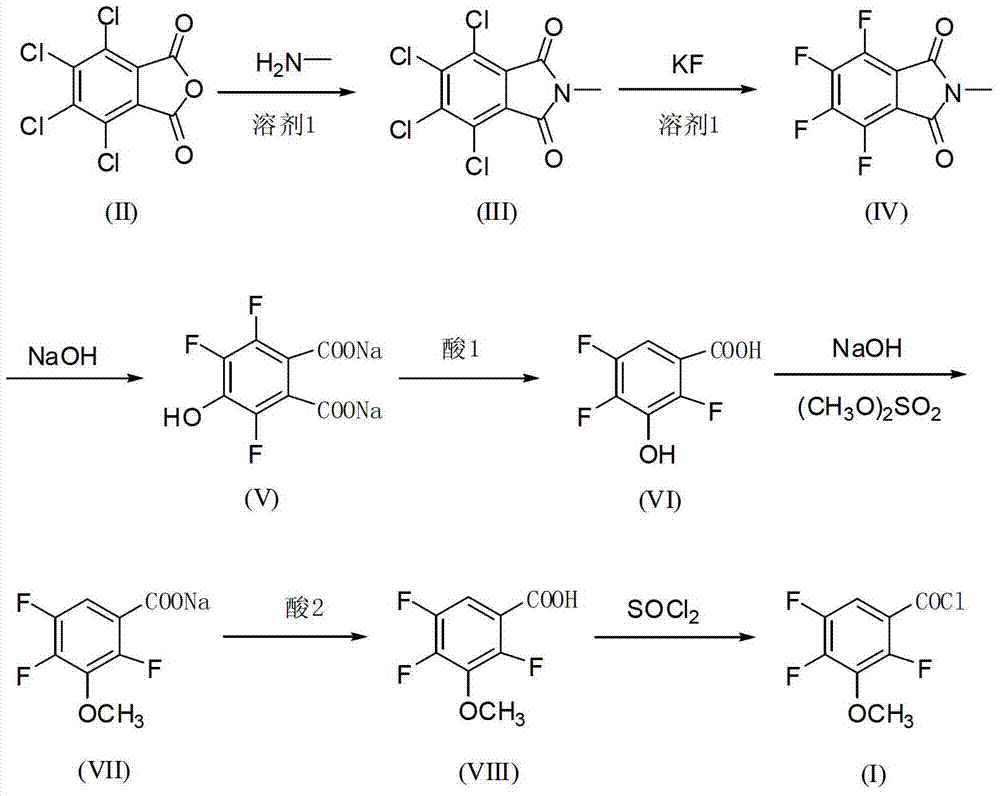
[0044] The preparation method of the 2,4,5-trifluoro-3-methoxybenzoyl chloride of the present embodiment comprises the following steps:
[0045] The first step: pump 1200kg of DMSO aqueous solution with a water content of 45-50% into a 2000L imidization kettle, add 300kg of tetrachlorophthalic anhydride, and add 85kg of monomethylamine aqueous solution (40% by weight) dropwise at a temperature below 35°C. Heat up to 105-110°C for reflux reaction for 10 hours, then cool down to 20-30°C for centrifugation, and dry the solid to obtain 310kg of N-methyltetrachlorophthalic imide. The mother liquor can be directly recycled;
[0046] Step 2: Pump 4000kg of anhydrous DMSO into a 5000L fluorination kettle, put in 900kg of anhydrous KF and 1000kg of N-methyltetrachlorophthalic imide, raise the temperature to 145-150°C for 2 hours, and then cool down to 70 °C, transfer to another kettle, add water to precipitate crystals, and centrifuge to obtain 950kg water-containing 20% N-methyltetr...
Embodiment 2
[0053] The preparation method of the 2,4,5-trifluoro-3-methoxybenzoyl chloride of the present embodiment comprises the following steps:
[0054] The first step: 1300kg of DMF aqueous solution containing 45-50% water is pumped into the 2000L imidization kettle, 300kg of tetrachlorophthalic anhydride is put in, and 85kg of monomethylamine aqueous solution (40% by weight) is added dropwise at a temperature below 35°C. Heat up to 105-110°C for reflux reaction for 10 hours, then cool down to 20-30°C for centrifugation, and dry the solid to obtain 308kg of N-methyltetrachlorophthalic imide. The mother liquor can be directly recycled and reused;
[0055] The second step: pump 4000kg of anhydrous DMF into the 5000L fluorination kettle, put in 920kg of anhydrous KF and 1000kg of N-methyltetrachlorophthalic acid imide, raise the temperature to 145~150℃ for 2 hours, and then cool down to 70 ℃, transferred to another kettle, added water to precipitate crystals, and centrifuged to obtain 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com