Azithromycin composition for injection and preparation method thereof
A technology of azithromycin and its composition, which is applied in the field of azithromycin composition for injection and its preparation, can solve the problems of harsh process conditions, increased cost, and easy to be decomposed and destroyed, and achieve high bioavailability, rapid reconstitution, and stable properties Effect
Inactive Publication Date: 2013-12-25
YOUCARE PHARMA GROUP
View PDF9 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
For example, patent CN200510014275.1 uses hydrochloric acid and lactobionic acid as cosolvents. Since azithromycin is insoluble in water, it is unstable under strong acid (hydrochloric acid, sulfuric acid) conditions and is easily decomposed and destroyed during the preparation process. The process conditions are also very harsh, resulting in The cost of azithromycin freeze-dried agent remains high
Patent CN95106702.8 uses lactic acid and acetic acid as cosolvents to prepare lactic acid and azithromycin acetate salts, but the solubility of azithromycin acetate salts decreases after being heated at 105 for 5 hours, and the color of azithromycin lactic acid salts will de
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
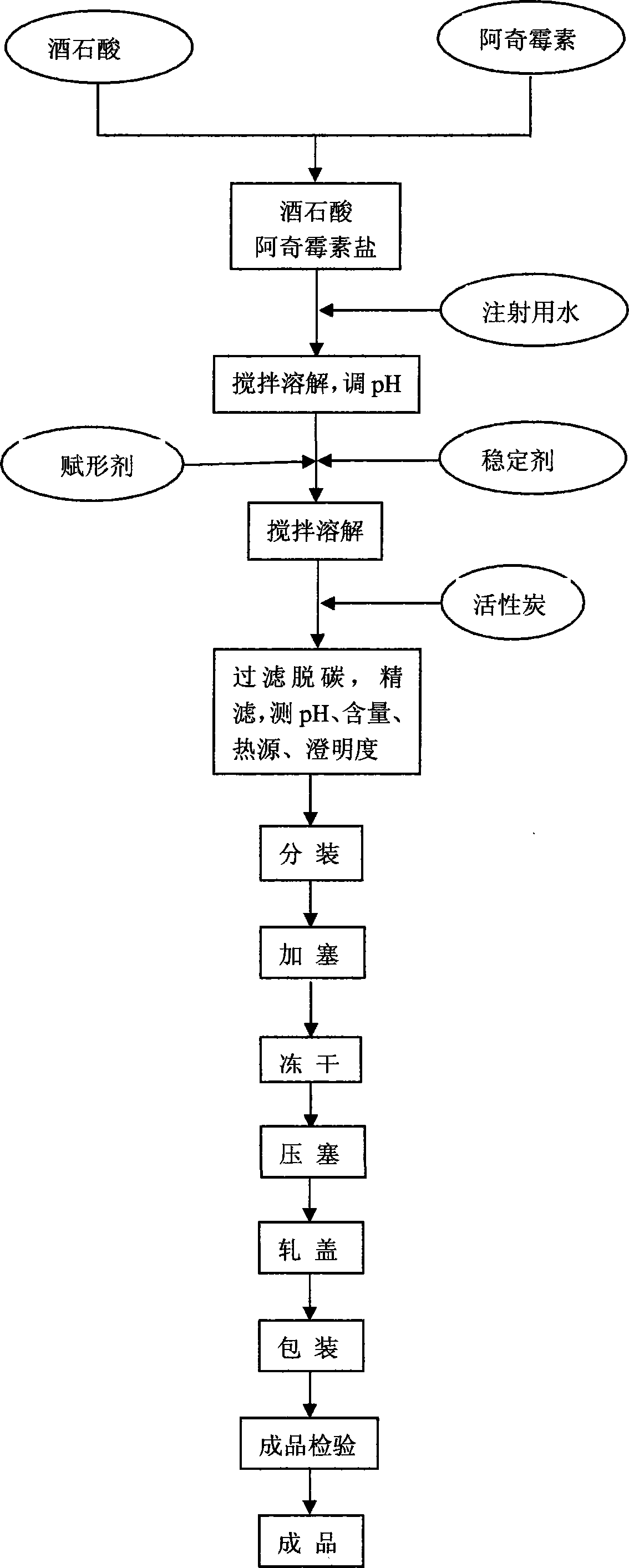
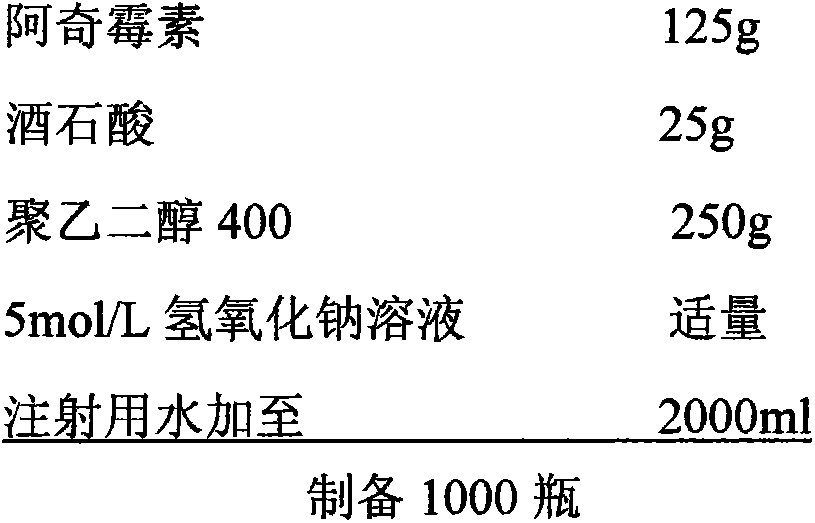
The invention relates to an azithromycin composition for injection and a preparation method thereof. The composition consists of azithromycin, organic acid/sodium salt, freeze-drying excipient and a stabilizer in a weight ratio of (10-60):(2-15):(0-50):(0-50). According to a chemical reaction method, azithromycin and organic acid react to prepare a water soluble salt, so that the solubility of the azithromycin is increased, and the stability of the composition is improved. The preparation method comprises the following steps of: preparing the azithromycin into the water soluble salt, adding water to dissolve the water soluble salt, regulating pH, adding and dissolving the freeze-drying excipient and the stabilizer, then adding active carbon for discoloring, filtering and decarburizing, and sub-packaging after refined filtration; cooling to -40 DEG C within 0.5 hour, preserving the heat and freezing for 3 hours, vacuumizing to 13.33Pa, gradually increasing to -10 DEG C from -40 DEG C within 14 hours, and continuously drying for 5 hours at 35 DEG C. the composition prepared by the preparation method disclosed by the invention is accurate in dosage, stable in quality and high in purity.
Description
technical field [0001] The invention relates to the field of pharmaceutical preparations, in particular to a composition of azithromycin for injection and a preparation method thereof. Background of the invention [0002] Azithromycin is the first new azolide antibiotic researched and developed by Pliva Company of Yugoslavia Croatia. It was launched in Yugoslavia in 1988 and in the United States in 1992, and then it was promoted worldwide. [0003] Azithromycin is a derivative of macrolide antibiotic erythromycin, which is effective for respiratory tract infection, urinary tract infection, skin infection, soft tissue infection and infection in other parts of the human body. Compared with erythromycin, azithromycin has a wider antibacterial spectrum, high bioavailability, long half-life, less frequent administration, and less gastrointestinal irritation. [0004] Azithromycin is the first preparation of a new type of azolide antibiotics. Its mechanism of action is the same a...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/19A61K31/7052A61K47/12A61K47/18A61P31/04
Inventor 李琦杨磊
Owner YOUCARE PHARMA GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com