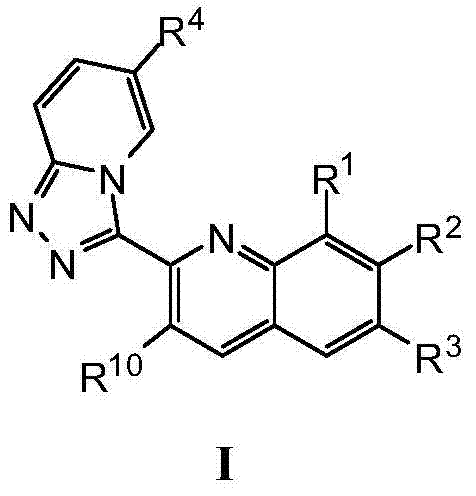
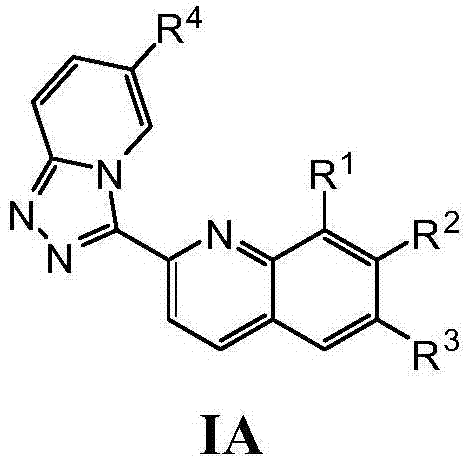
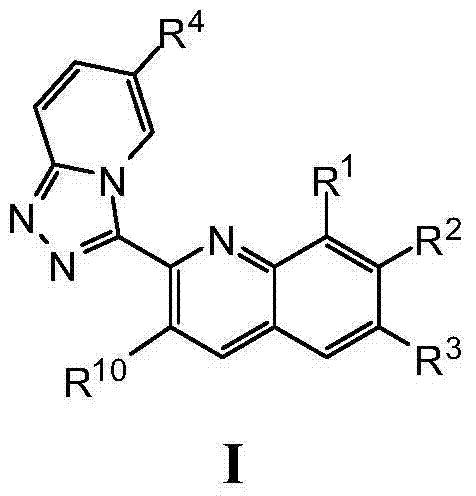
Triazolopyridine compounds as PIM kinase inhibitors
A technology of compound and solvate, applied in the field of manufacturing said compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0399] The following examples illustrate the invention. In the examples described below, all temperatures are expressed in degrees Celsius unless otherwise stated. Reagents were purchased from commercial suppliers such as Aldrich Chemical Company, Acros, Lancaster, TCI or Maybridge unless otherwise stated and were used without further purification unless otherwise stated. Unless otherwise stated, tetrahydrofuran (THF), dichloromethane (DCM), toluene and dioxane were purchased from Aldrich in Sure sealed vials and used as received.
[0400] The reactions described below are typically carried out in anhydrous solvents under positive pressure of nitrogen or argon or with drying tubes (unless otherwise stated), and the reaction flasks are usually fitted with rubber septa for introduction of substrates and reagents via syringes . Glassware is oven-dried and / or heat-dried. Column chromatography was performed on a Biotage system (Manufacturer: Dyax Corporation) with a silica gel c...
Embodiment A
[0403] The assay used to measure PIM-1 activity is based on the 33 P]ATP[ 33 P]PO 4 PIM2tide substrate was incorporated and radiolabeled peptides were captured on Whatman P81 (phosphocellulose) filter plates. Subsequently, the amount of radiolabeled product was determined by liquid scintillation counting. Final buffer conditions were as follows: 20 mM K + MOPS (pH 7.4), 10 mM MgCl 2 , 0.005% Tween-20, 1 mM DTT. The assay mixture contains 35 μM [γ- 33 P]ATP (20 μCi / mL), 7.5 μM PIM2tide and 0.25 nM PIM-1. Incubate for 60 min at 22°C with 75 μL of 200 mM H 3 PO 4 Quenched, filtered through Whatman P81 plates, and washed with 200 mM H 3 PO 4 Wash (1 x 200 [mu]L and 5 x 100 [mu]L). Subsequently, 50 μL of liquid scintillation mix was added to each well, and the plate was counted for 30 sec / well using TopCount NXT.
[0404] IC 50 Assay:
[0405] Compounds were prepared by 3-fold serial dilutions from 500-μM intermediate dilutions at ...
Embodiment B
[0407] PIM-2 determination
[0408] As described in Example A, 4 μM [γ- 33 P]ATP (20 μCi / mL), 1.0 μM PIM2tide and 1.5 nM GST-labeled recombinant full-length human Pim-2 were assayed in place of PIM-1. Average IC of compounds tested in this assay 50 Values are provided in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com