Freeze-drying process of insulin
An insulin and freeze-drying technology, used in drying, drying solid materials, drying solid materials without heating, etc., can solve the problems of long drying time, residual organic solvent, affecting the biological activity and purity of insulin, and achieve stable quality, The effect of reducing impurity content and eliminating adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
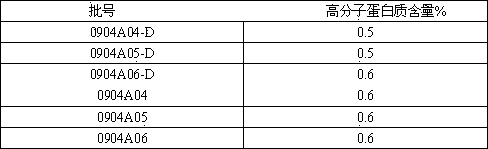
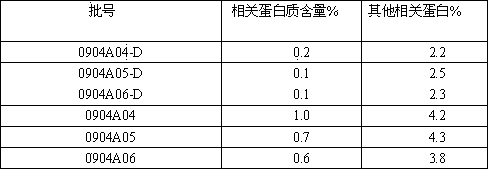
[0031] The freeze-drying process of insulin comprises the following steps:
[0032] a. Pretreatment of insulin crystals to be lyophilized;
[0033] b. Turn on the freeze dryer, adjust the temperature in the freeze drying room to below -25°C, and put the pretreated insulin crystals into the freeze dryer for rapid freezing;
[0034] c. Adjust the temperature in the freeze-drying chamber to -15°C, keep the temperature in the freeze-drying chamber at -15±2°C, and pre-freeze the product for 1 to 2 hours;
[0035] d. Adjust the temperature of the condenser to -40±2°C, then adjust the temperature of the freeze-drying chamber to 0°C gradually, and return the temperature to no more than 2°C per hour, under vacuum conditions, and keep it for 1~2 hours;
[0036] e. Continue to adjust the temperature of the freeze-drying chamber to 15°C, and the temperature should not exceed 7.5°C per hour, and maintain the temperature of the freeze-drying chamber at about 15°C, under vacuum conditi...
Embodiment 2
[0039] On the basis of Example 1, in step d, the vacuum condition is 18.0±8.0pa.
Embodiment 3
[0041] On the basis of the above examples, in the step e, the vacuum condition is 15.0±5.0pa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com