Chromene compound
一种色烯化合物、键合的技术,应用在有机化学、变色荧光材料、化学仪器和方法等方向,能够解决未获知色烯化合物等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0156]
[0157] The preparation method of the chromene compound of the present invention is not particularly limited, and any synthesis method can be used.
[0158] The chromene compound represented by the above formula (1) can be efficiently produced, for example, by the following method.
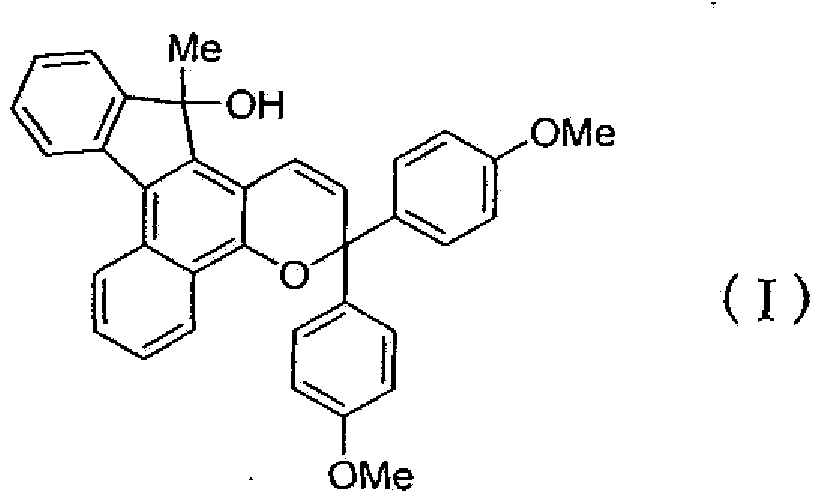
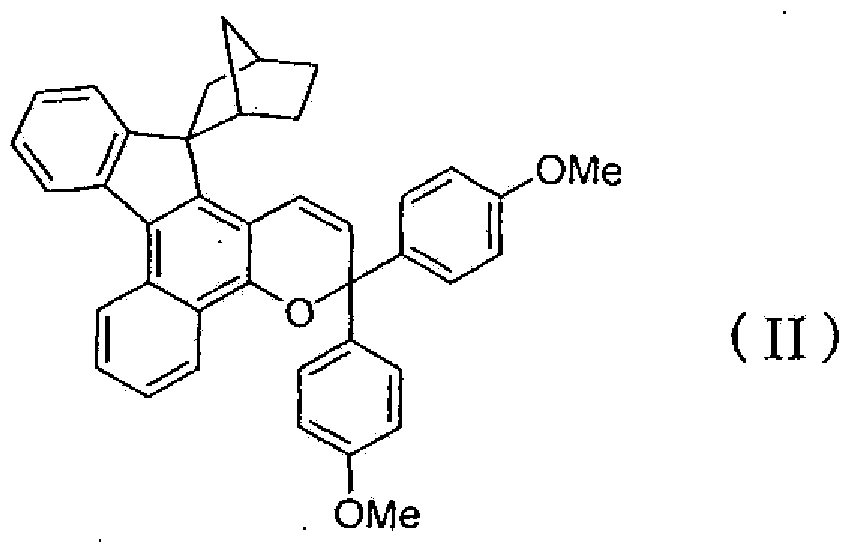
[0159] That is, it can be produced by a method of reacting a naphthol compound represented by the following formula (6) with a propargyl alcohol compound represented by the following formula (7) in the presence of an acid catalyst.
[0160]
[0161] In the formula, R 1 ,X,A,C * and a are the same as defined in the above formula (1).
[0162]
[0163] (where, R 2 and R 3 Same definition as in formula (1) above).
[0164] The reaction ratio of the naphthol compound and the propargyl alcohol compound can be used in a wide range, and is preferably selected from the range of 1:10 to 10:1 (molar ratio). In addition, as the acid catalyst, for example, sulfuric acid, benzenesulfonic ...
Embodiment 1
[0209] Embodiment 1 (synthesis of chromene compound E1)
[0210] (Synthesis of naphthol compounds)
[0211] Add 50.0g (172mmol) of 3-bromo-4-methoxybenzophenone and 28.7g (189mmol) of 4-methoxyphenylboronic acid into 250ml of 1,2-dimethoxyethane, and add 25 ml of ethanol, 400 g of a 10% aqueous sodium carbonate solution, and 0.05 g (0.043 mmol) of tetrakis(triphenylphosphine)palladium were added and reacted at 78°C. After 3 hours, 1,000 ml of toluene was added to the reaction solution, the organic layer was washed with water, and the solvent was removed, followed by recrystallization with 200 ml of methanol to obtain the following formula as a white solid:
[0212]
[0213] The indicated 4-methoxy-3-(4-methoxyphenyl)benzophenone was 51.6 g (162 mmol, yield 94%).
[0214] This benzophenone compound and 32.5 g (186 mmol) of diethyl succinate were dissolved in 150 ml of tetrahydrofuran. A tetrahydrofuran solution of 23.6 g (211 mmol) of potassium tert-butoxide was dropped i...
Embodiment 2
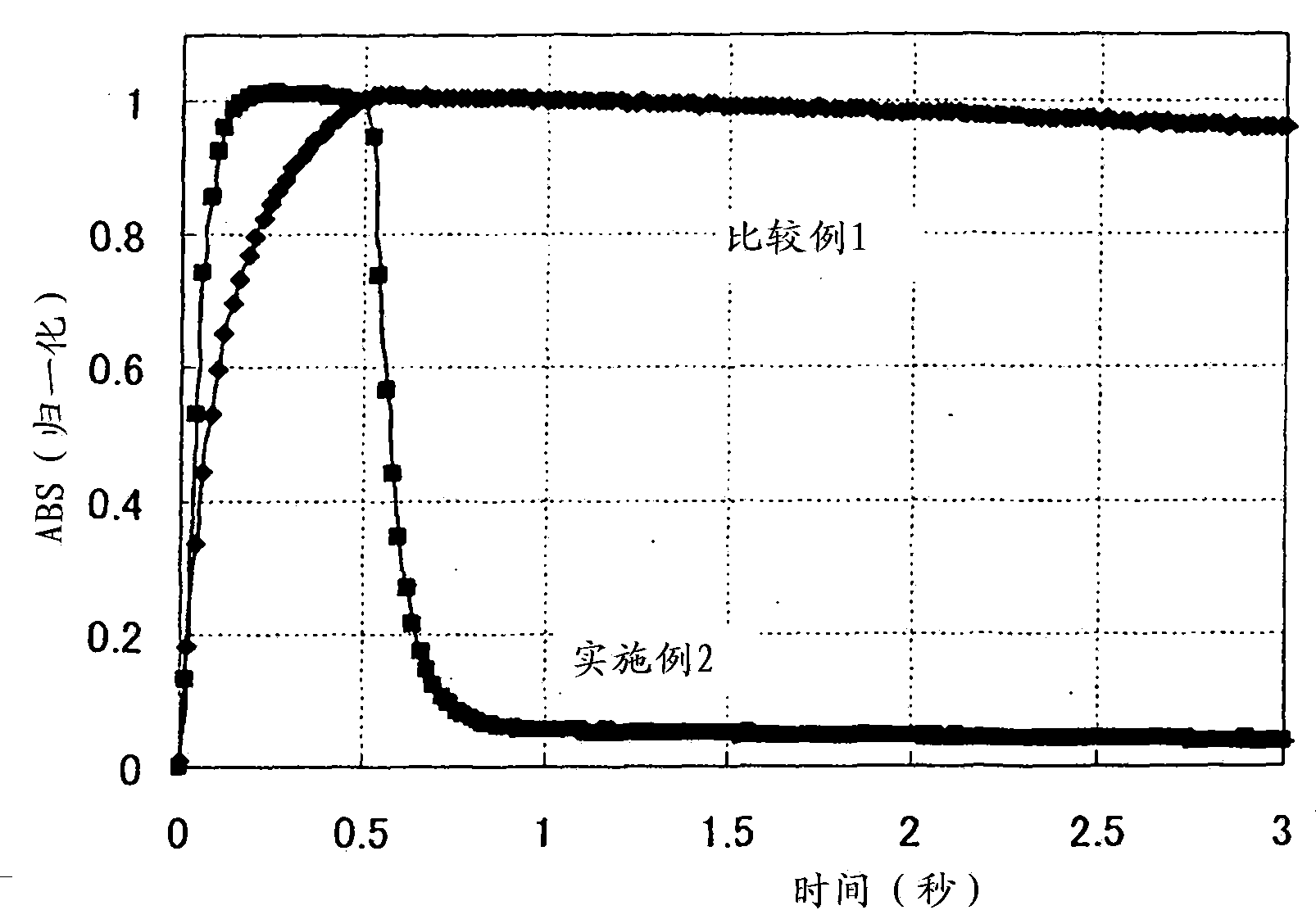
[0243] Example 2 (Evaluation of Photochromic Properties in Solution)
[0244] The chromene compound (E1) obtained in Example 1 was dissolved in tetrahydrofuran to prepare a concentration of 0.5 mM. The solution was added to a quartz colorimetric cell with an optical path length of 1 mm as a sample. The temperature of the sample is 23°C ± 1°C, and the UV-LED irradiator manufactured by Omron Corporation (ZUV-C20H for the controller part, ZUV-H20MB for the irradiation head unit part, and ZUV-L8H for the lens unit part) is used as the light source. The distance between the sample and the light source is 50mm, irradiate 365nm ultraviolet light to make it color, and measure the photochromic characteristics. The photochromic properties were evaluated by the following items.
[0245] [1] Maximum absorption wavelength (λmax): It is the maximum absorption wavelength after color development obtained by a spectrophotometer (momentary multi-channel photodetector MCPD2000M) manufactured b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| UV absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com