Proline sulfinamide double-chirality organic molecular catalyst and preparation method thereof
A technology of proline sulfenamide and proline formamide trifluoroacetate, which is applied in the field of bichiral organic small molecule catalysts and its preparation, and achieves the effects of easy-to-obtain raw materials, simple operation and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
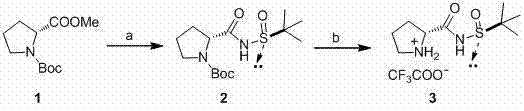
[0036] 1.( R )-N-(( S )-tert-butylsulfinyl) 2-prolinecarboxamide: under ice-water bath, add 480 mg (12 mmol, 60%) NaH to a dry 150-ml three-necked flask filled with nitrogen, add 25 ml of freshly distilled THF, rapidly injected with 1.21 g (10 mmol) of ( S)-tert-butylsulfinamide in 15 milliliters of anhydrous THF, after half an hour of reaction, slowly add 2.39 grams (10 mmol) of N-tert-butoxycarbonyl-D-proline methyl ester dropwise with a dropping funnel, drop After the addition was complete, remove the ice-water bath and react for 48 hours. Add saturated ammonium chloride solution to quench the reaction, extract with ethyl acetate (30 ml × 3), combine the organic phases, dry with anhydrous sodium sulfate, spin off the solvent, spin dry and separate by silica gel column chromatography (petroleum ether: Ethyl acetate=3:1), afforded 2a (2.38 g) as a white solid. Yield 75%.
[0037] ( R )-N-(( R )-tert-butylsulfinyl) 2-proline carboxamide: under ice-water bath, filled wit...
Embodiment 2
[0040] Embodiment two: catalyst ( R )-N-(( S )-tert-butylsulfinyl) 2-proline carboxamide trifluoroacetate, ( R )-N-(( R )-tert-butylsulfinyl)2-prolinecarboxamide trifluoroacetate was applied to the direct asymmetric aldol condensation reaction respectively, 3a catalyst can be synthesized in high yield and high diastereoselectivity and enantioselectivity A-hydroxy ketone compound, reaction principle is as follows:
[0041]
[0042] The catalyst is:
[0043]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap