A method for synthesizing high-purity δ-layered sodium disilicate from silicon tetrachloride
A technology of silicon tetrachloride and sodium disilicate, applied in the direction of silicate, chlorine/hydrogen chloride, alkali metal silicate, etc., to achieve simple production process, high utilization value, good calcium and magnesium ion exchange capacity and buffer capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
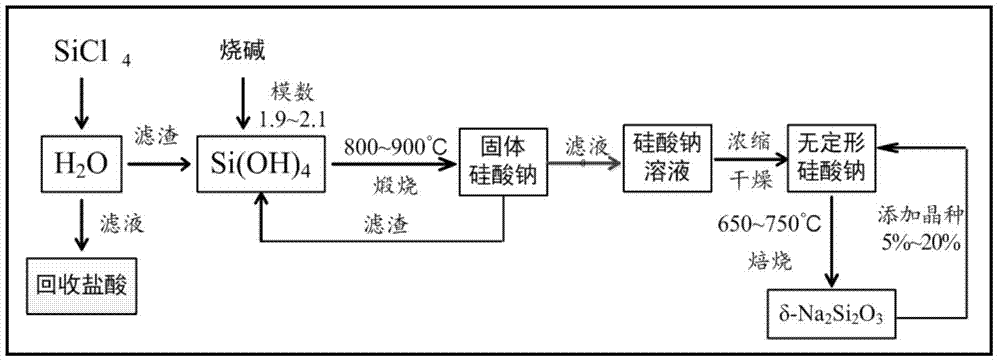
[0027] (A) Take 31.4ml of pure water and place it in a 150ml Erlenmeyer flask, and evenly drop 10ml of silicon tetrachloride into the pure water with an autosampler at a rate of 1.5ml / min. The molar ratio is 1:20 (4 times excess water);
[0028] (B) Separate the product of step (A) with a suction filtration device, recover the filtrate as hydrochloric acid after concentration, and transfer the filter residue (ortho silicic acid) to a 50ml nickel crucible;
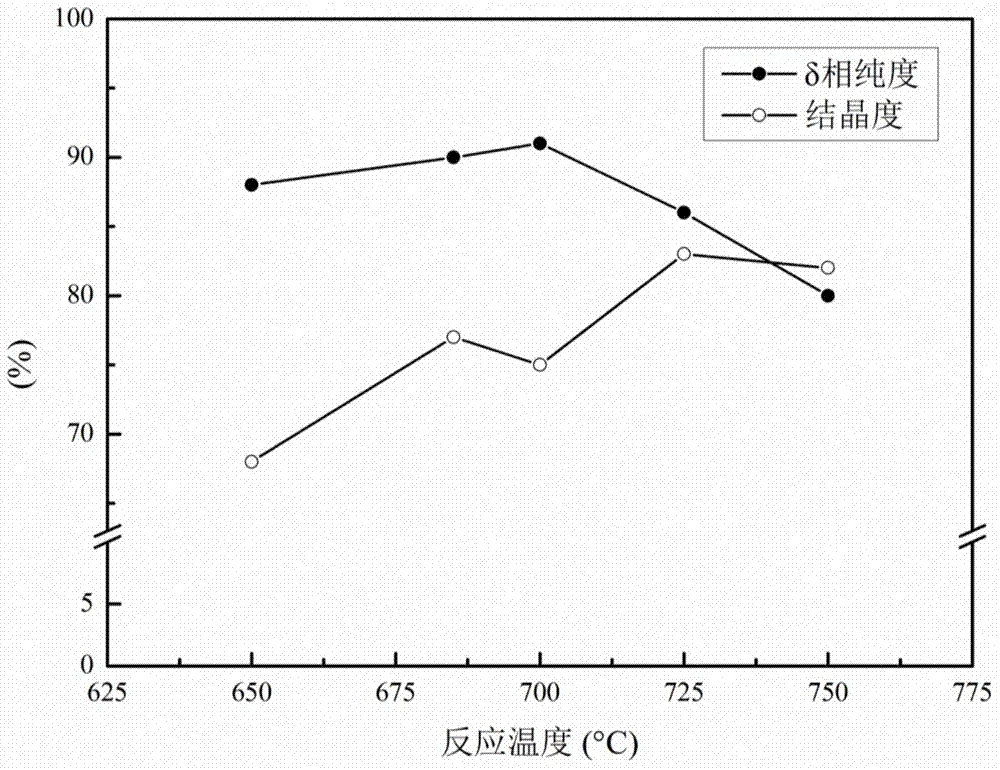
[0029] (C) Weigh 3.48g of NaOH solid and add it into a 50ml nickel crucible so that SiO 2 / Na 2 The O modulus is 1.9-2.1; after mixing the reactants, place the nickel crucible in a muffle furnace at 850°C for calcination for 1 hour, take out the nickel crucible and cool it to obtain solid sodium silicate;
[0030] (D) Take 15ml of pure water and add it to a 50ml beaker, heat to 80°C, transfer the solid sodium silicate in step (C) to the beaker to dissolve; use a suction filter to separate the product, filter residue (unre...
Embodiment 2
[0034] (A) Take 12.56ml of pure water and place it in a 150ml Erlenmeyer flask, and evenly drop 10ml of silicon tetrachloride into the pure water with an autosampler at a rate of 1ml / min. The moles of silicon tetrachloride and water The ratio is 1:8 (1 times excess water);
[0035] (B) Separate the product of step (A) with a suction filtration device, recover the filtrate as hydrochloric acid after concentration, and transfer the filter residue (ortho silicic acid) to a 50ml nickel crucible;
[0036] (C) Weigh about 3.48g of NaOH solid and add it into a 50ml nickel crucible so that SiO 2 / Na 2 The O modulus is 1.9-2.1; after mixing the reactants, place the nickel crucible in a muffle furnace at 800°C for calcination for 2 hours, take out the nickel crucible and cool it to obtain solid sodium silicate;
[0037] (D) Take 15ml of pure water and add it to a 50ml beaker, heat to 70°C, transfer the solid sodium silicate in step (C) to the beaker to dissolve; use a suction filter t...
Embodiment 3
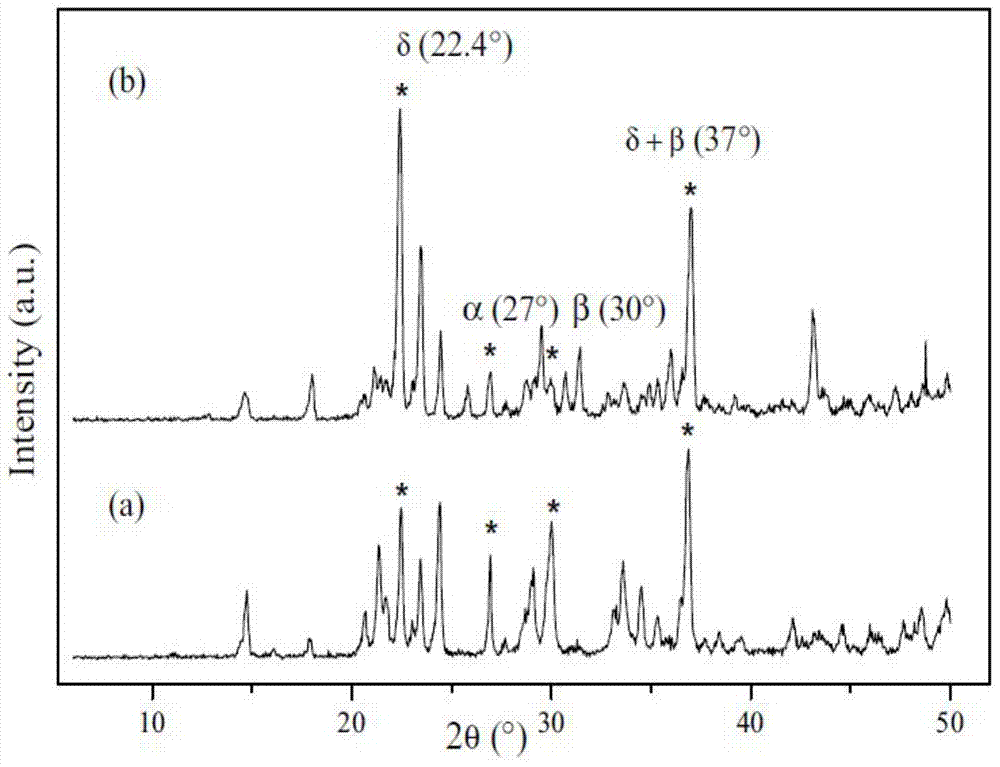
[0041] The steps are the same as in Example 1, except that the high-temperature calcination temperature in step (F) is 650°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com