Resorufin-2,4-dinitrophenyl ether and its application in the detection of thiophenol
A technology of dinitrophenyl ether and resorufin, which is applied in the field of UV-Vis spectrophotometry for the determination of thiophenol and the new compound resorufin-2,4-dinitrophenyl ether, which can solve the problem of operation Cumbersome, expensive, inconvenient for popularization and application, etc., to achieve the effect of simple operation, good selectivity and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of resorufin-2,4-dinitrophenyl ether
[0019] Add 0.15g (0.64mmol) resorufin sodium, 8mL dry DMF, 0.3mL triethylamine to a 25mL round bottom flask, stir at 40°C for 30 minutes, and dissolve 0.16g (0.79mmol) 2,4-dinitrochlorobenzene Dissolve in 2mL of dry DMF, then add dropwise into a round bottom flask, then add 0.1477g (0.90mmol) KI, stir at 80°C for 12 hours, after the reaction is complete, mix the solution with 50mL CH 2 Cl 2 Dilute, wash in sequence with distilled water (20mL×3), wash with brine (20mL×3), and dry under reduced pressure to obtain the crude product, which is purified by column chromatography, and the eluent is the volume ratio of ethyl acetate to petroleum ether For a 1:1 mixed solution, 0.1 g of yellow solid resorufin-2,4-dinitrophenyl ether was obtained, with a yield of 41%, and the structural formula was as follows
[0020]
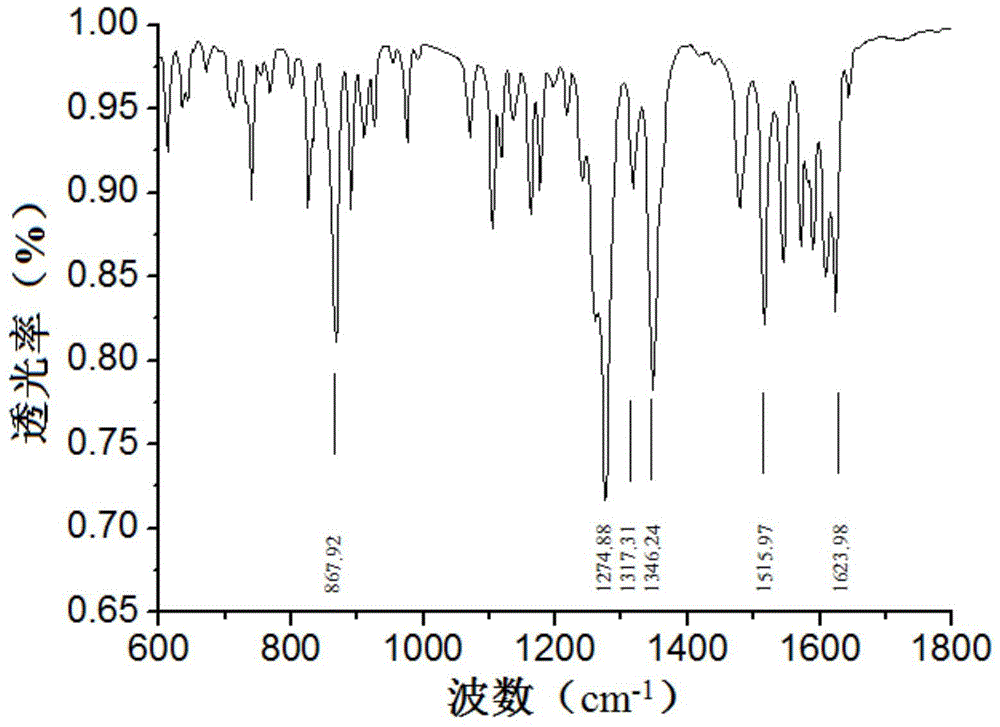
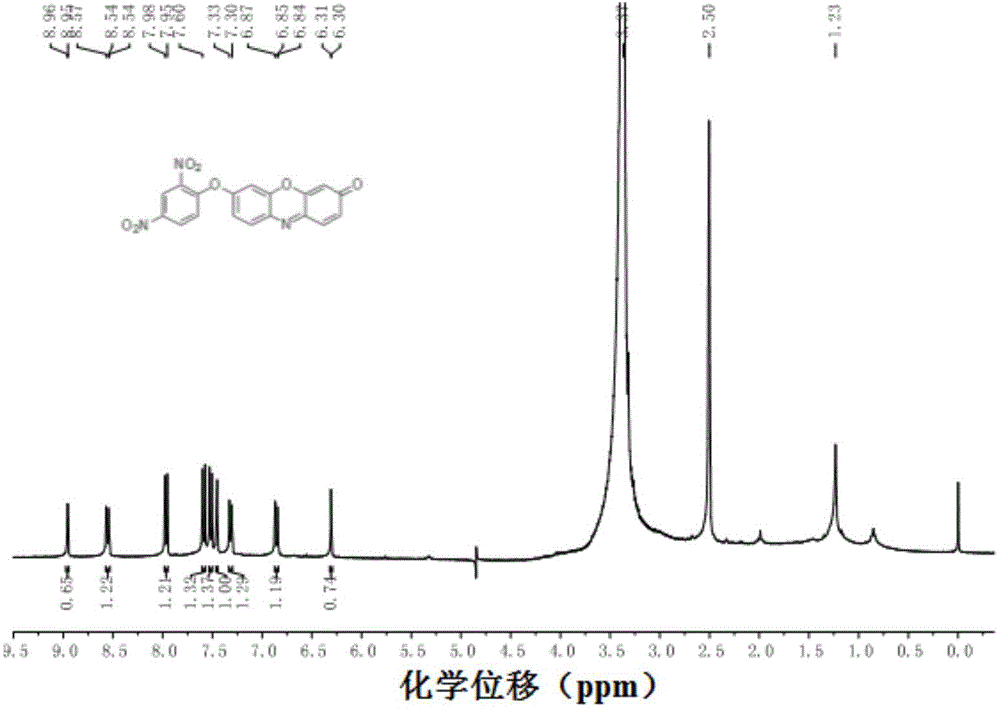
[0021] Infrared spectra and 1 The H-NMR diagrams are shown in figure 1 with figure 2 , the specific spe...
Embodiment 2
[0025] The use of the compound resorufin-2,4-dinitrophenyl ether prepared in Example 1 in the detection of thiophenol, its specific detection method is as follows:
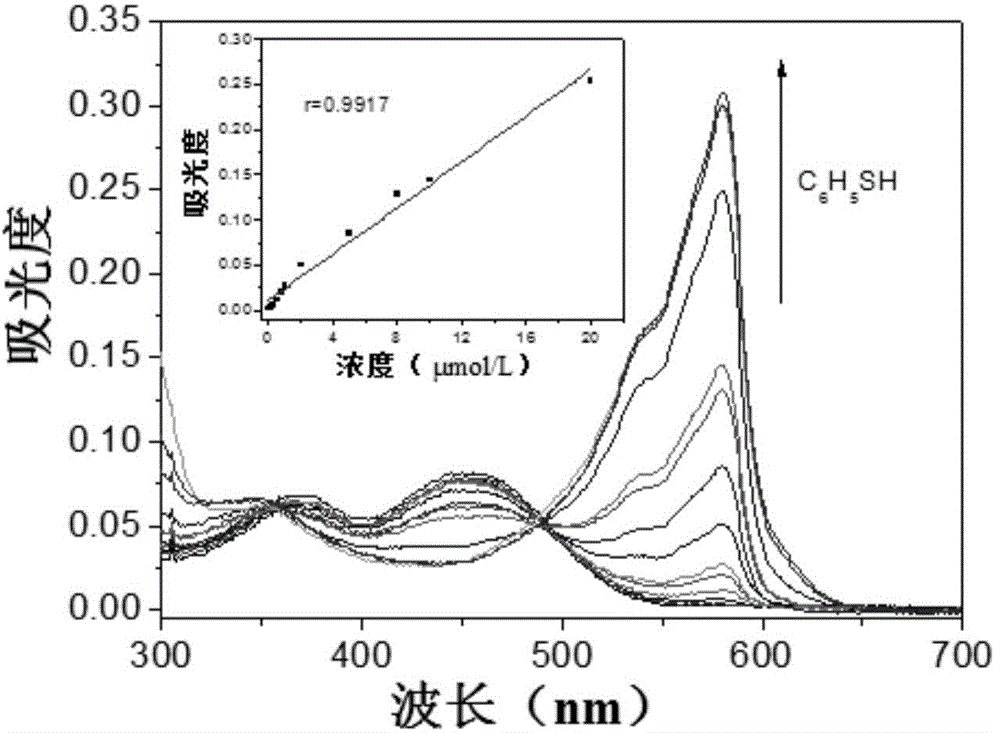
[0026] Add 4.5mL of absolute ethanol, 2.0mL of phosphate buffer solution with a pH value of 7.4, 0.05mL of 1.0mmol / L resorufin-2,4-dinitrophenyl ether to a 10mL colorimetric tube in sequence. Sulfone solution and the sample solution to be tested were fixed to volume with sub-boiling water, left to stand at room temperature for 20 minutes, and the absorbance of the reaction system was detected with a UV-visible spectrophotometer at a wavelength of 580nm, and calculated according to the formula A=0.01272C+0.01167 The concentration of thiophenol in the sample solution, where A is the absorbance value of the reaction system at 580nm, and C is the concentration of thiophenol in the reaction system, in μmol / L.
[0027] In order to prove the beneficial effects of the present invention, the inventor has carried out a larg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com