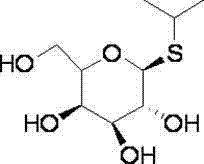
Isopropyl-beta-D-thiogalactoside preparation method
A technology of thiogalactoside and propylthioacetyl, which is applied in the field of preparation of isopropyl-β-D-thiogalactoside, achieves easy availability of raw materials, improves safety factor, saves operating costs and materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] a) Add 5.5mol acetic anhydride and 1mol aluminum trichloride at room temperature, add 1mol galactose in 9 batches at 5-10°C, add 1.1mol isopropyl mercaptan after the reaction is completed, and obtain 0.765mol isopropyl mercaptan after the reaction is completed Propylthioacetylgalactose;
[0027] b) Add 0.765 mol of isopropylthioacetylgalactose to 10 mol of methanol to dissolve, add 0.01 mol of sodium methoxide, add 0.01 mol of acetic acid to neutralize after the reaction is completed, and obtain 0.743 mol of isopropyl-β-D- Thiogalactosides. The yield was 74.3%.
[0028] The post-treatment in step a is to dropwise add 10 mol of water at 0-5°C and stir for 2 hours, extract the aqueous layer with 5 mol of dichloromethane, separate the organic phase and wash the organic phase with 10 mol of water three times, separate the organic phase and concentrate, add 1mol tert-butyl methyl ether and 2mol isohexane mixed solution crystallized, filtered and dried.
[0029] The post-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com