Hydrosilylation reaction inhibitors, and use thereof for preparing stable curable silicone compositions
A technology of hydrosilylation and hydropolysiloxane, which is applied in the directions of compositions for inhibiting chemical changes, chemical instruments and methods, synthetic resin layered products, etc., can solve problems such as unsatisfactory solutions, etc., Achieving the effect of large bath stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
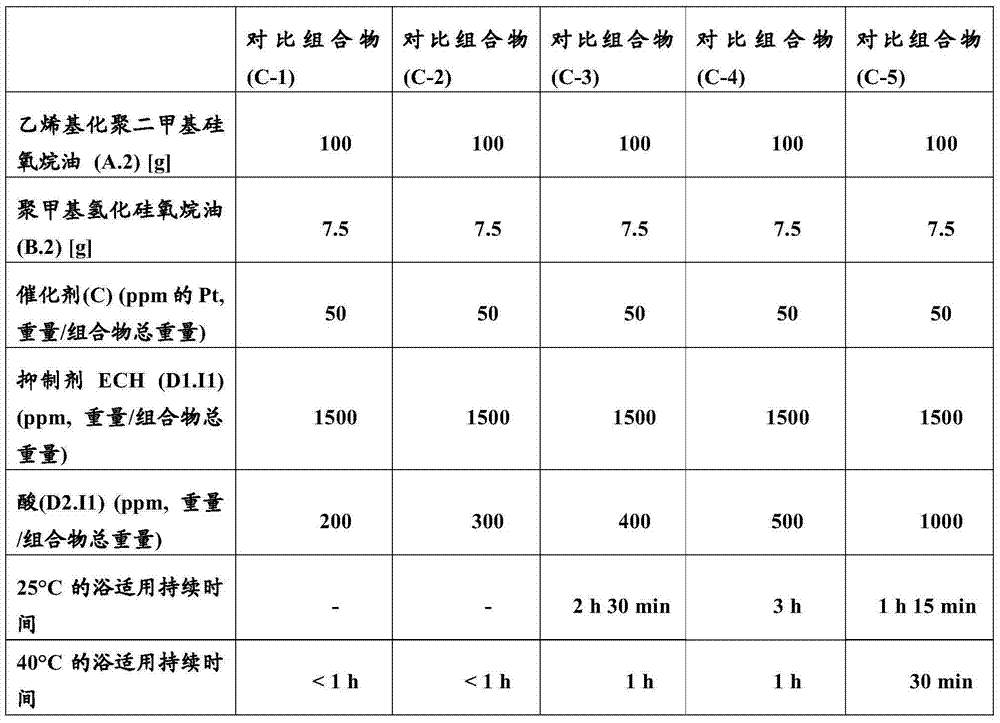
[0100] The inhibitor ECH (D1.I1) was pre-added to the vinylated dimethicone oil (A.1). After homogenization of the mixture, the polymethylhydrosiloxane oil (B.1) is introduced, then the amount of acid to be tested and finally the catalyst (C). The time required for crosslinking at ambient temperature (25°C) and 40°C is measured = bath application duration (durée de vie) at 25°C and 40°C. The results are reported in Table 1 below.
[0101] Table 1
[0102]
[0103] [H / vinyl] molar ratio = 1.8
[0104] It was observed that when the amount of platinum catalyst was 50 ppm relative to the total weight of the composition, the presence of variable amounts of methanesulfonic acid (D2.I1) was not able to obtain a satisfactory bath service duration, especially at 40 ℃.
Embodiment 2
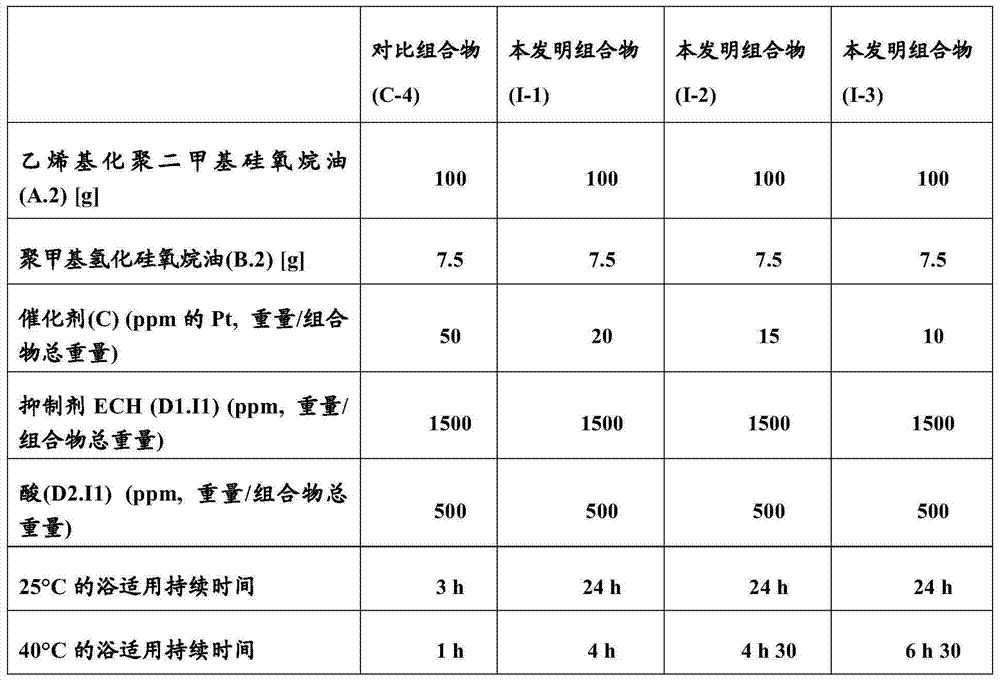
[0106] The inhibitor ECH (D1.I1) was pre-added to the vinylated dimethicone oil (A.1). After homogenization of the mixture, polymethylhydridosiloxane oil (B.1) is introduced, followed by a fixed amount of 500 ppm (relative to the total weight of the composition) of the acid to be tested and finally a variable amount of catalyst (C) . Measure the time required for crosslinking at ambient temperature (25°C) and 40°C = bath application duration at 25°C and 40°C. The results are reported in Table 2 below.
[0107] Table 2
[0108]
[0109] [H / vinyl] molar ratio = 1.8
[0110] It can be observed that the test according to the invention (platinum content of less than 50 ppm by weight relative to the total weight of the composition) makes it possible to obtain a very good bath service duration at 25° C. of about 24 hours instead of 3 hours . At 40°C, the improvement of the composition according to the invention is about 400% to 650%.
Embodiment 3
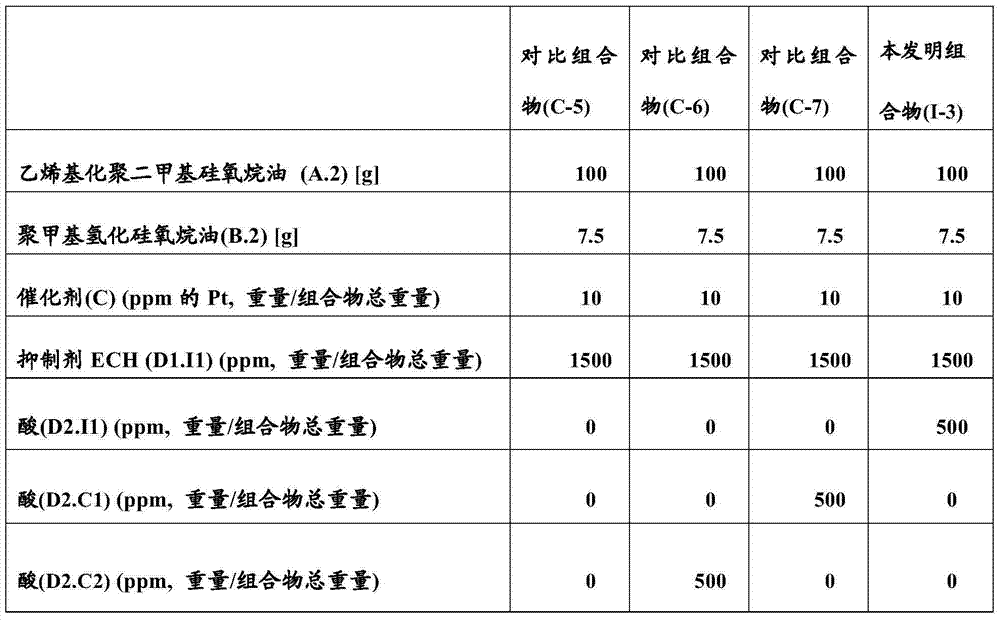
[0112] The inhibitor ECH (D1.I1) was pre-added to the vinylated dimethicone oil (A.1). After homogenization of the mixture, polymethylhydridosiloxane oil (B.1) is introduced, then the acid to be tested, and finally, the amount of catalyst (C) is fixed at 10 ppm of platinum relative to the total weight of the composition . The formulations are reported in Table 3 below.
[0113] table 3
[0114]
[0115] A sample of each composition was extracted and analyzed by DSC ("Differential Scanning Calorimetry", METLER type device). The analysis was performed in aluminum pans and using a temperature rampe of 25-250°C with a gradient of 10°C / min. This technique is also capable of measuring the peak onset temperature (start of the crosslinking reaction) or "T°C onset", peak apex temperature ("T°C peak") and peak end temperature ("T°C end" of each composition). ).
[0116] Thermal profiles, characteristic data of exothermic peaks (T°C onset, T°C peak and T°C end) are shown in Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com