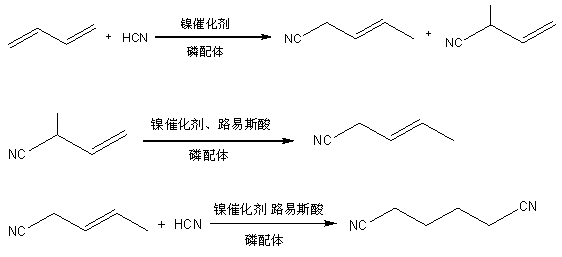
Method for synthesizing adiponitrile by isomerization liquid of 2-methyl-3-crotononitrile
A technology of isomerization and butene nitrile, applied in the field of synthesis of adiponitrile, can solve the problems of separation difficulty, increase of equipment cost and production cost, separation and purification of 3PN increase process flow and process complexity, etc., so as to reduce equipment cost and Production cost, effect of reducing poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Add 2-methyl-3-butenenitrile, tri-o-cresyl phosphite, zero-valent nickel catalyst and aluminum trichloride into the isomerization reactor at a molar ratio of 50:10:1:1, and control The reaction pressure is 0.1Mpa, the reaction temperature is 100°C, and the heat preservation reaction is carried out for 8 hours to obtain the isomerization liquid; the isomerization liquid is analyzed for 3-pentenenitrile, 2-methyl-3-butenenitrile, tri-o-phosphite The mol ratio of cresyl ester, zero-valent nickel catalyst and aluminum chloride is 48:2:10:1:1;
[0024] 2) Add the isomerization solution and hydrocyanic acid obtained in step 1) into the secondary hydrocyanation reaction kettle, the molar ratio of hydrocyanic acid to 3-pentenenitrile in the isomerization solution is 1:1, and the reaction is controlled The pressure is 0.3Mpa, the reaction temperature is 80°C, and the heat preservation reaction is carried out for 4 hours. After the reaction is completed, the adiponitrile produ...
Embodiment 2
[0026] 1) Add 2-methyl-3-butenenitrile, tri-o-cresyl phosphite, zero-valent nickel catalyst and aluminum trichloride into the isomerization reactor at a molar ratio of 100:15:1:5, and control The reaction pressure is 0.1Mpa, the reaction temperature is 100°C, and the heat preservation reaction is carried out for 8 hours to obtain the isomerization liquid; the isomerization liquid is analyzed for 3-pentenenitrile, 2-methyl-3-butenenitrile, tri-o-phosphite The mol ratio of cresyl ester, zero-valent nickel catalyst and aluminum chloride is 95:5:15:1:5;
[0027] 2) Add the isomerization solution and hydrocyanic acid obtained in step 1) into the secondary hydrocyanation reaction kettle, the molar ratio of hydrocyanic acid to 3-pentenenitrile in the isomerization solution is 1:3, and the reaction is controlled The pressure is 0.3Mpa, the reaction temperature is 80°C, and the heat preservation reaction is carried out for 4 hours. After the reaction is completed, the adiponitrile prod...
Embodiment 3
[0029] 1) Add 2-methyl-3-butenenitrile, tri-o-cresyl phosphite, zero-valent nickel catalyst and aluminum trichloride into the isomerization reactor at a molar ratio of 80:10:1:2, and control The reaction pressure is 0.1Mpa, the reaction temperature is 100°C, and the heat preservation reaction is carried out for 8 hours to obtain the isomerization liquid; the isomerization liquid is analyzed for 3-pentenenitrile, 2-methyl-3-butenenitrile, tri-o-phosphite The mol ratio of cresyl ester, zero-valent nickel catalyst and aluminum chloride is 76:4:10:1:2;
[0030] 2) Add the isomerization solution and hydrocyanic acid obtained in step 1) into the secondary hydrocyanation reaction kettle, the molar ratio of hydrocyanic acid to 3-pentenenitrile in the isomerization solution is 1:5, and the reaction is controlled The pressure is 0.3Mpa, the reaction temperature is 80°C, and the heat preservation reaction is carried out for 4 hours. After the reaction is completed, the adiponitrile produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com