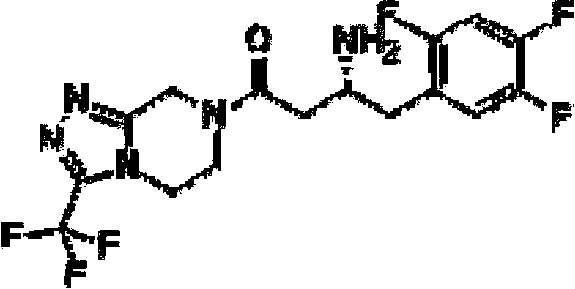
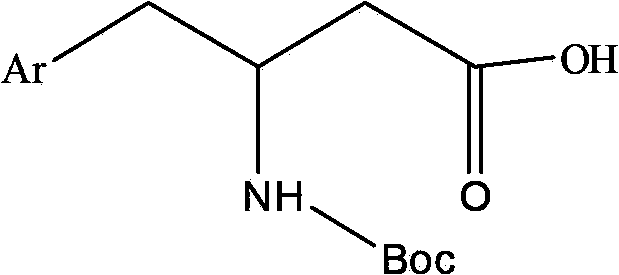
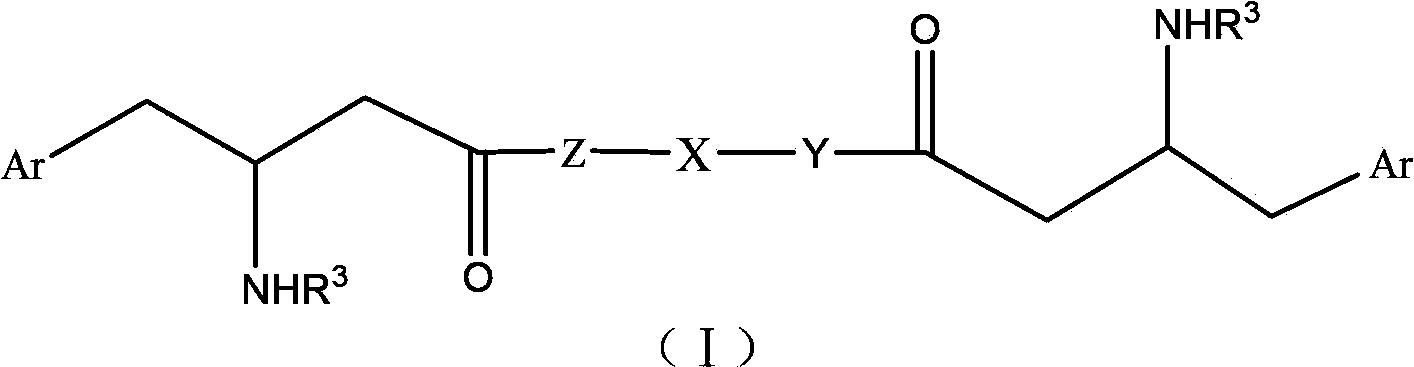DPP-4 (Dipepitidyl Peptidase-4) inhibitor compound
A compound and selected technology, applied in the field of DPP-4-related diseases, dipeptidyl peptidase IV (DPP-4) inhibitor compounds, can solve GLP-1 inactivation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]
[0074] experiment procedure:
[0075] first step:
[0076] Method A
[0077] Weigh Boc-(R)-3-amino-4-(2,4,5-trifluorophenyl)butyric acid (1.0g, 3.0mmol), anhydrous piperazine (0.26g, 3.0mmol), HBTU( 2.27g, 6.0mmol) was added to a 100ml round bottom flask, dissolved with 20ml of DMF, then DIEA (0.78g, 6.0mmol) was added to the reaction solution, and the reaction was stirred at room temperature for 1.5 hours. TLC detection (petroleum ether: ethyl acetate = 4:1), the reaction is over, the reaction solution is added to 10 times the amount of water, a white solid precipitates out, filtered, the filter cake is washed with water, and the solid obtained is dried.
[0078] Method B
[0079] Weigh Boc-(R)-3-amino-4-(2,4,5-trifluorophenyl)butyric acid (1.0g, 3.0mmol), anhydrous piperazine (0.26g, 3.0mmol), DCC( 6.0mmol) and HoBt (6.0mmol) were added to a 100ml round bottom flask, dissolved in 20ml DMF, and then DIEA (12.0mmol) was added to the reaction solution, and the reaction was st...
Embodiment 2
[0085] Chemical reaction formula:
[0086]
[0087] Experimental process: The specific experimental process of compound 2 is similar to that of Example 1, refer to Example 1.
[0088] MSm / z(ESI): 613.5[M+Na]
[0089] 1 H-NMR (500MHz, deuterated DMSO): δ7.31~7.38(m,2H), 7.13~7.22(m,2H), 4.01~4.15(m,2H), 4.42(m,1H), 3.67(s ,3H), 2.80~2.91(m.4H), 2.59~2.72(m,4H).
Embodiment 3
[0091] Chemical reaction formula:
[0092]
[0093] Experimental process: The specific experimental process of compound 3 is similar to that of Example 1, refer to Example 1.
[0094] MSm / z(ESI): 539.3[M+H]
[0095] 1 H-NMR (500MHz, deuterated DMSO): δ7.60~7.65(m,4H), 7.31~7.38(m,2H), 7.13~7.22(m,2H), 4.42(m,1H), 4.01~4.15 (m, 2H),, 3.67(s, 3H), 2.80~2.91(m.4H), 2.59~2.72(m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com