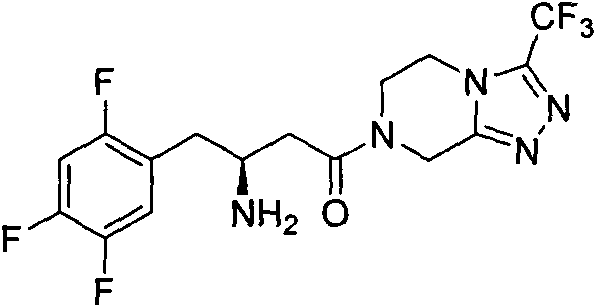
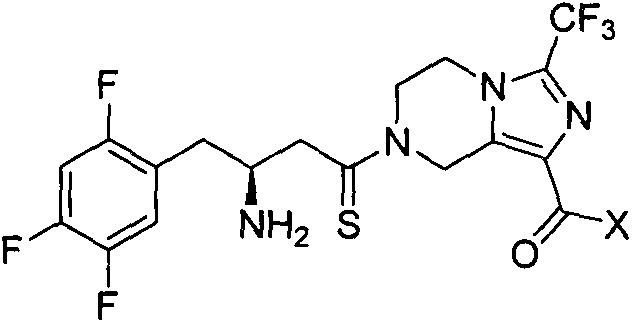
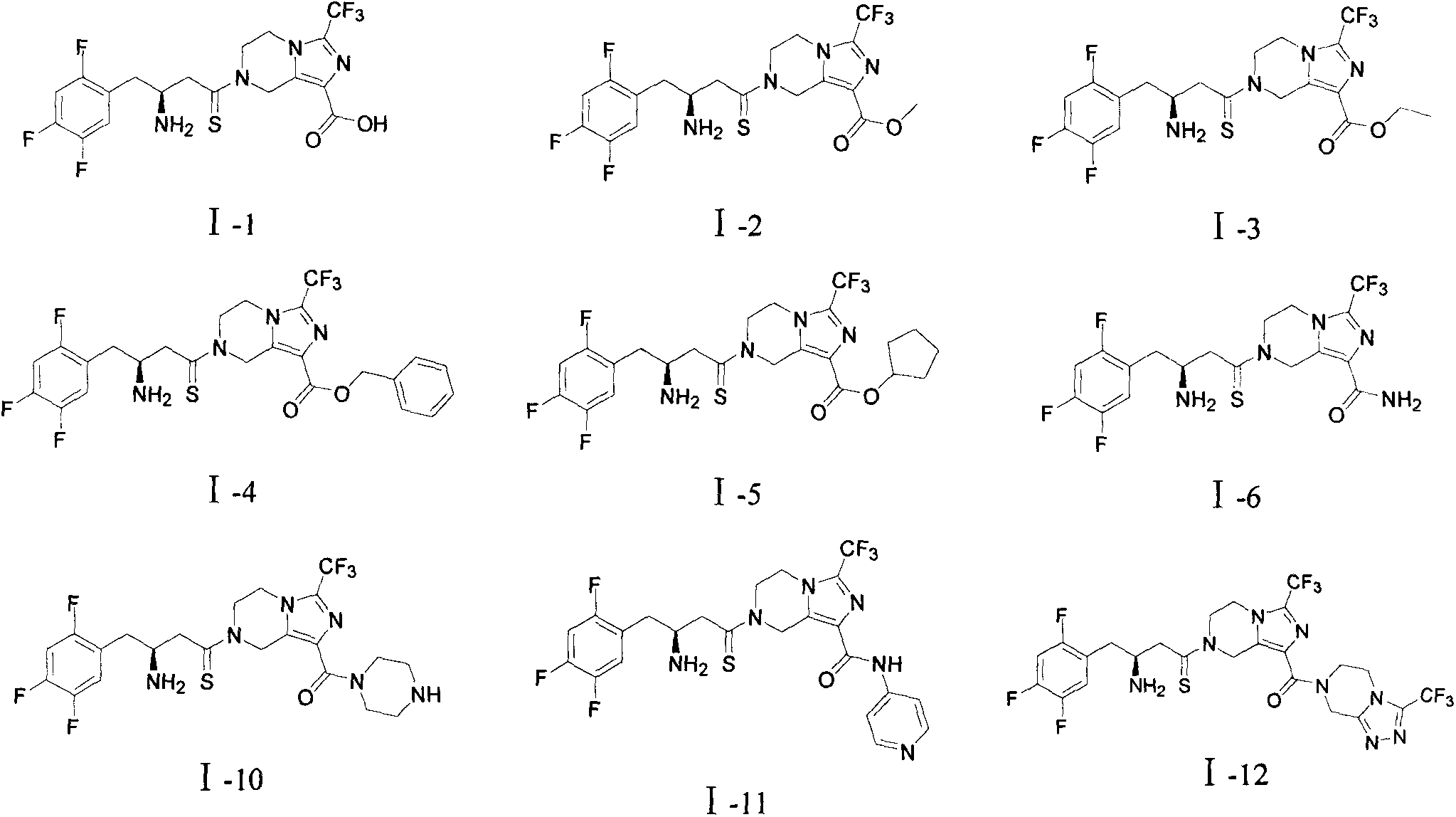
Piperazidines derivate
A compound and pharmaceutical technology, applied in the field of dipeptidyl peptidase IV inhibitor compounds and DPP-4-related diseases, can solve the problems of not meeting clinical needs, limited varieties of diabetes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 key intermediate formula II
[0059] Chemical reaction formula:
[0060]
[0061] In step (a) to step (f), select compound 1 (C-pyrazin-2-yl-methylamine) as the starting material to prepare compound 8. The specific experimental steps refer to the seventh step in Example 1 of the invention patent CN101824036A to the preparation method of the twelfth step.
[0062] Step (g):
[0063] Add 0.17g of compound 8 into a 100ml single-necked bottle, add 10ml of toluene to dissolve it, then add 0.07g of LR, reflux at 120°C overnight, TLC (PE: EA = I: 1) the reaction of the raw material is complete, evaporate the solvent, and purify by column chromatography , and evaporated to dryness to obtain 0.12 g of compound 9.
[0064] Step (h):
[0065] Add 8.8g NaOH and 180ml of methanol to a 250ml single-necked bottle successively, stir at room temperature to dissolve, add 2.5g of compound 9, heat the oil bath to reflux, TLC (PE:EA=2:1, 2 drops of ace...
Embodiment 2
[0067] The preparation of embodiment 2 compound I-1
[0068] Add about 40 ml of methanol-hydrogen chloride solution (pH≈3) into the single-necked bottle containing 0.6 g of the compound of formula II, and stir overnight at room temperature. TLC (DCM:MeOH=20:1, 2 drops of ammonia) tracked the completion of the reaction, and the reaction solution was concentrated to dryness under reduced pressure to obtain 0.42 g of a yellow solid, namely compound I-1. MS: 481 [M+H].
[0069] H-NMR: δ7.42-7.34(m, 1H), 7.25-7.23(m, 1H), 5.18-5.07(m, 2H), 4.37-4.32(m, 1H), 4.15-4.00(m, 2H) , 3.96-3.92 (m, 2H), 3.26-2.89 (m, 2H), 2.84-2.80 (m, 2H).
Embodiment 3
[0070] The preparation of embodiment 3 compound I-7
[0071] Chemical reaction formula:
[0072]
[0073] Add 0.98g of compound of formula II, 15ml of DMF, 0.75g of triethylamine, 1g of HOBT and 1.41g of EDC·HCl into a 100ml three-necked flask, stir at room temperature for 1h, add 1.0ep of tetrahydrofuran solution of methylamine, and react at 31°C overnight. TLC (DCM: MeOH=20:1, 2 drops of ammonia) followed the completion of the reaction, the reaction solution was poured into 200ml of ice water, a large amount of white solid was precipitated, filtered with suction, the filter cake was washed with water, and dried in vacuum at 45°C to obtain 0.8g of a gray solid, namely is compound 10.
[0074] Referring to the method of Example 2, compound 10 was de-boc-protected to obtain compound I-7.
[0075] MS: 480 [M+H].
[0076] H-NMR: δ7.43-7.35 (m, 1H), 7.26-7.24 (m, 1H), 5.18-5.06 (m, 2H), 4.36-4.31 (m, 1H), 4.16-4.02 (m, 2H), 3.97-3.92 (m, 2H), 3.24-2.87 (m, 2H), 2.87-2.82 (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com