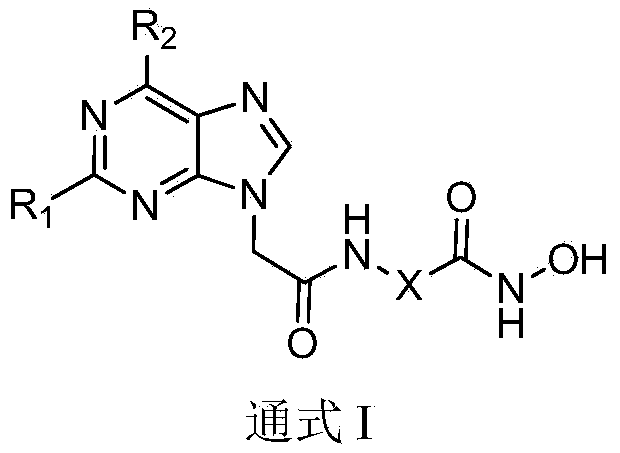
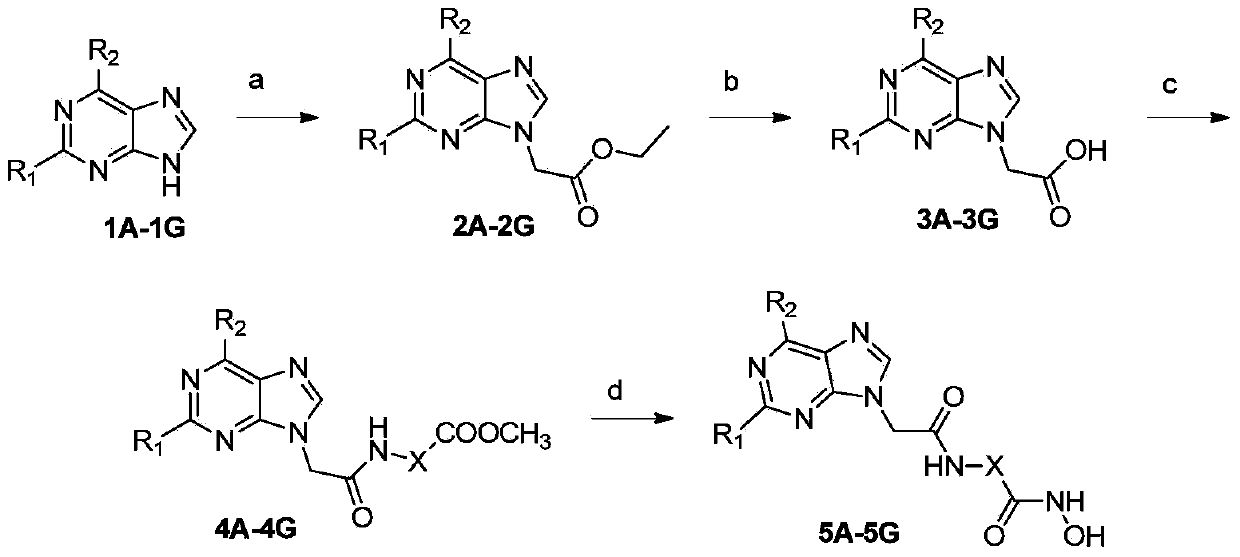
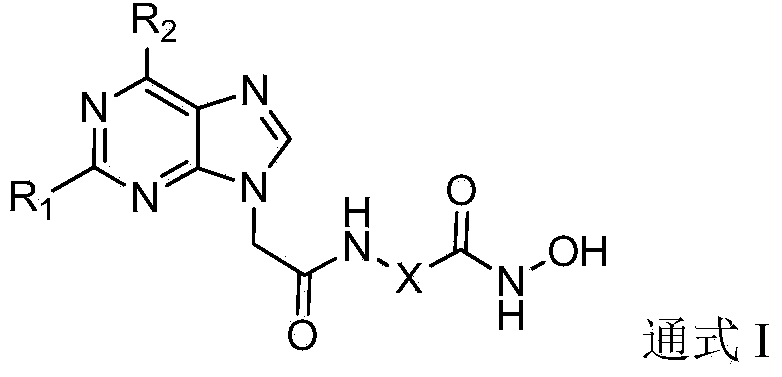
Substituted purin-9-acetamino isohydroxamic acid histone deacetylase inhibitor and preparation method and application thereof
A technology of acetylaminohydroxime and sirtuin, which is applied in the field of medicine and can solve problems such as toxicity, heart disease, and clinical application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0094] Example 1. Synthesis of 2-[2-(6-benzylamine-9H-purin-9-yl)acetamido]-N-hydroxyacetamide (5A1)
[0095] Synthesis of ethyl 2-(6-benzylamino-9H-purin-9-yl)acetate (2A)
[0096] Compound 6-benzylamine-9H-purine (1A, 6.76g, 30mmol) was dissolved in 50mL DMSO, and K 2 CO 3 (12.4g, 90mmol) and ethyl chloroacetate (4mL, 36mmol), react at room temperature for 4h. After the reaction, 5 times the volume of water was added, then extracted with ethyl acetate, dried over magnesium sulfate, concentrated, and recrystallized at P / E=1:2 to obtain a white solid (6.07g, 65%). m.p.182-183℃; 1 H NMR (400MHz, DMSO-d 6 )δ:1.22(t,3H,J=7.2Hz),4.18(q,2H,J=7.2Hz),4.77(br s,2H),5.08(s,2H),7.20–7.38(m,5H) ,8.14(s,1H),8.20(s,1H),8.25(s,1H).
[0097]Compounds 2B-2G were synthesized according to the method of 2A.
[0098] Synthesis of 2-(6-benzylamino-9H-purin-9-yl)acetic acid (3A)
[0099] Starting material 2A (4.67g, 15mmol) was dissolved in 30mL THF / MeOH (volume ratio 3:1), and 23mL of 2M N...
Embodiment 2
[0110] Example 2. Synthesis of 3-[2-(6-benzylamine-9H-purin-9-yl)acetamido]-N-hydroxypropionamide (5A2)
[0111] The preparation methods of intermediates and target compounds are as in Example 1. Yield 78%; m.p.201–202°C; 1 H NMR (300MHz, DMSO-d 6 )δ:2.15(t,J=7.2Hz,2H),3.25–3.29(m,2H),4.72(br s,2H),4.81(s,2H),7.18–7.22(m,1H),7.26– 7.35(m,4H),8.07(s,1H),8.16(s,1H),8.23(br s,1H),8.37(t,J=5.4Hz,1H),8.71(s,1H),10.41( s,1H); HRMS calcd for C 17 h 19 N 7 o 3 370.1622, found 370.1615.
Embodiment 34
[0112] Example 3. Synthesis of 4-[2-(6-benzylamine-9H-purin-9-yl)acetamido]-N-hydroxybutyramide (5A3)
[0113] The preparation methods of intermediates and target compounds are as in Example 1. Yield 84%; m.p.198-199°C; 1 H NMR (300MHz, DMSO-d 6 )δ:1.64(quint,J=7.2Hz,2H),1.98(t,J=7.2Hz,2H),3.08(q,J=6.0Hz,2H),4.72(br s,2H),4.82(s ,2H),7.18-7.23(m,1H),7.26-7.35(m,4H),8.08(s,1H),8.17(s,1H),8.27-8.30(m,2H),8.68(s,1H ),10.33(s,1H); HRMS calcd for C 18 h 21 N 7 o 3 384.1779, found 384.1785.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com