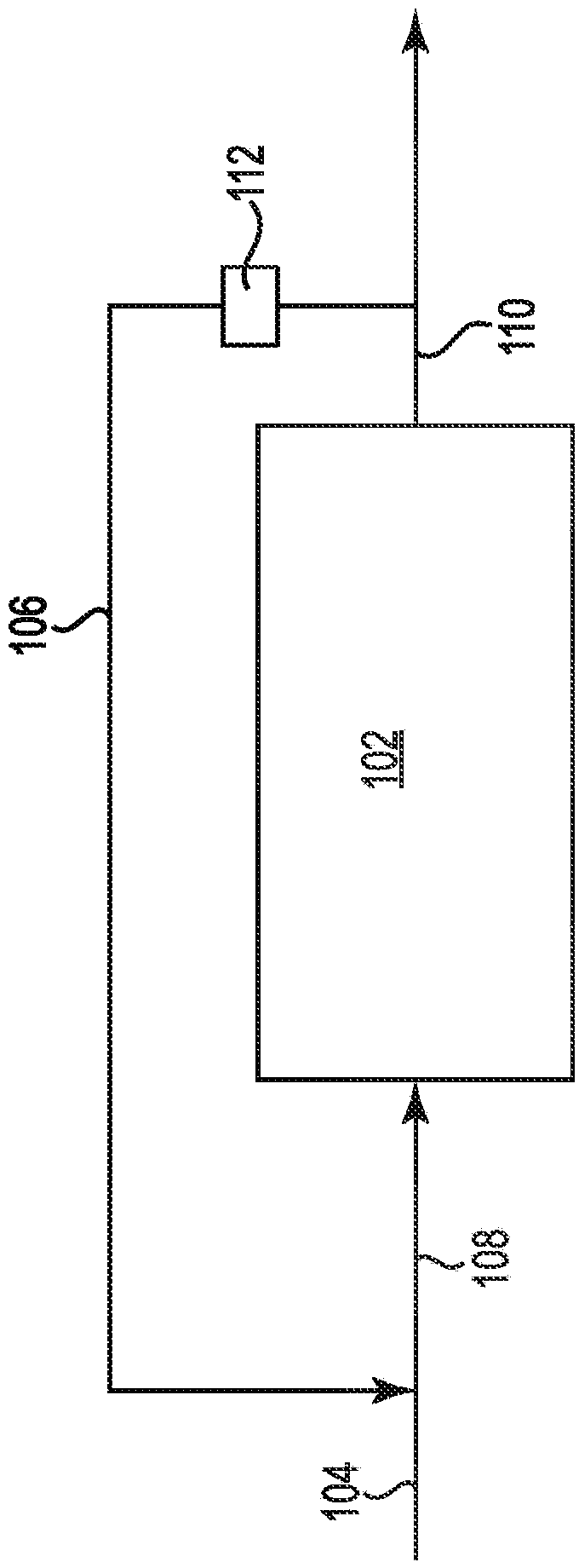
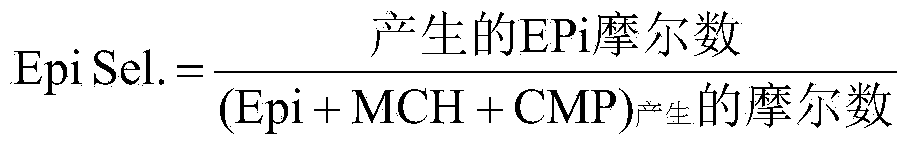Process and system for producing an oxirane
A technology for oxirane and peroxy compounds, which is applied in the field of production of oxirane compounds, and can solve problems such as high cost, reduced selectivity of oxirane compounds, energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
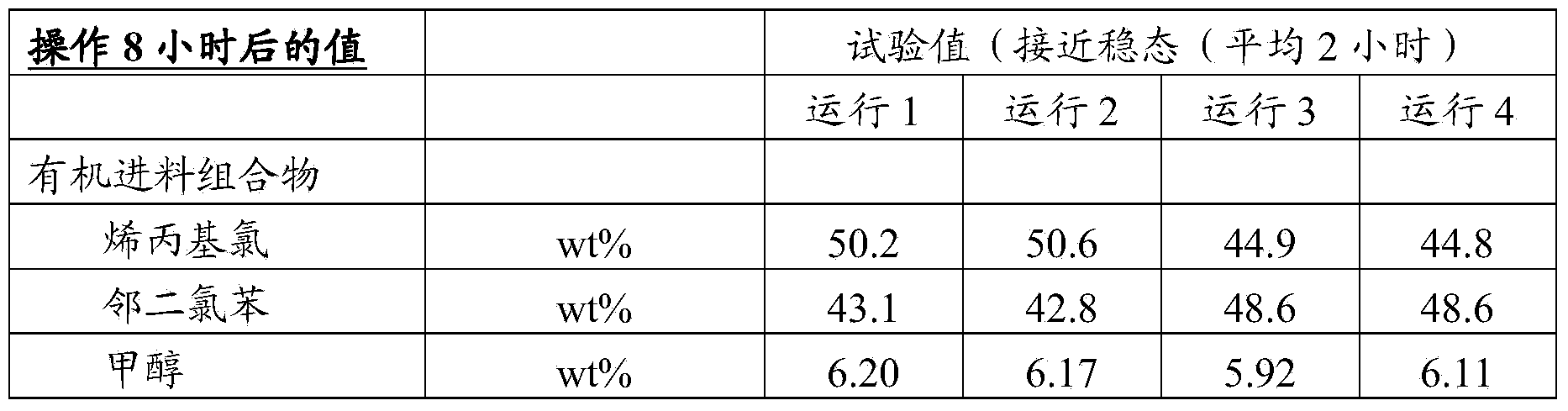
[0109] Example 1 (run 1)
[0110] The test covers the optimum working conditions that yield the highest selectivity in at least 8 hours of operation, and even up to 24 hours of operation. Reactor at temperature 10°C and space velocity 1.8hr -1 Under operation, the Epi selectivity obtained is 98.2%.
Embodiment 2
[0111] Example 2 (run 2)
[0112] This experiment was performed to illustrate the effect of temperature at similar LHSV values. The run was at a reactor temperature of 40 °C and a space velocity of 1.9 hr -1 Under operation, the Epi selectivity obtained is 83.2%. While the other two control parameters (such as productivity and reduction in peroxide concentration in the effluent) improved, the selectivity dropped dramatically.
Embodiment 3 and 4
[0113] Examples 3 and 4 (runs 3 and 4)
[0114] These examples are comparative examples to Example 2 as a means of demonstrating the effect of varying the LHSV when operating at the same reactor temperature of 40°C. Examples 2-4 cover the entire range of LHSVs claimed. It shows that as LHSV increases (from 0.48 to 14.7hr -1 ), the Epi selectivity is improved (98% of the mismatch cold temperature), and the H in the effluent 2 o 2 Increased concentration (e.g. decreased peroxide conversion).
[0115] pressure drop
[0116] Table 2 summarizes the pressure drop achieved across the catalyst bed in the reactor during the process run at the indicated reaction mixture composition and recycle stream flow rate.
[0117] Table 2 – Pressure drop
[0118]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com