Peripheral Modified Aggregation-Induced Luminescent Enhanced Dendrimer with Chromophore and Its Preparation Method and Application
A technology of aggregation-induced luminescence and chromophore, which is applied in the field of dendrimers and their preparation, can solve the problems of enhancing non-radiative decay process and restricting applications, and achieves the effect of suppressing non-radiative decay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
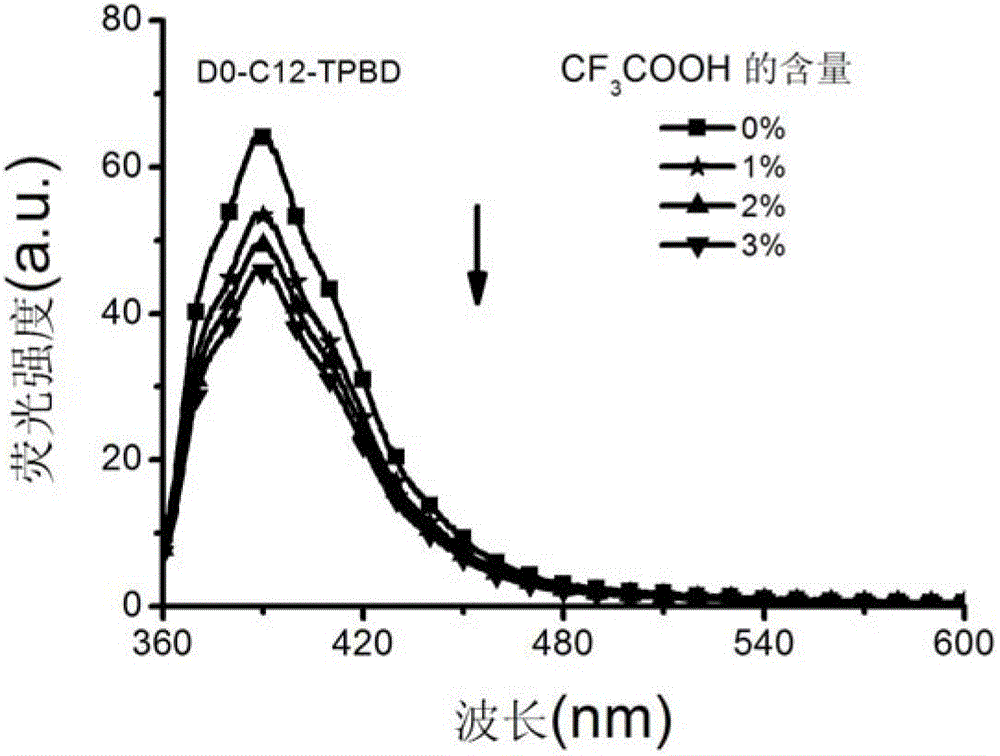
[0032] Preparation of 0-generation tetraphenylbutadiene (hereinafter referred to as: TPBD) chromophore-modified dendrimer (D0-C12-TPBD) with dodecyldiamine as the core
[0033]
[0034] Add 3mmol of tetraphenylbutadiene (TPBD) carboxylic acid derivative TPBD-COOH and 15mL of DMSO into a 50mL two-necked flask, and then add 4.2mmol of 1-ethyl-(3-dimethylaminopropyl)carbonyl Diimine hydrochloride, 3mmol 1-hydroxybenzotriazole (HOBT). Stir the reaction at room temperature under nitrogen protection for 2h, and slowly add 20mL of 0-generation solution with dodecyldiamine as the nucleus to the reaction system dropwise. The DMSO solution of polyamide-amine dendrimer (G0-C12), the ratio of the amount of TPBD chromophore to the peripheral amine group of G0-C12 is 1.4:1, continue to stir and react for 72h, and pour the reaction solution slowly into 300mL of water, filter and wash the solid with 30mL of water, and dry the crude product in a vacuum oven at 40°C. Dissolve the solid with...
Embodiment 2
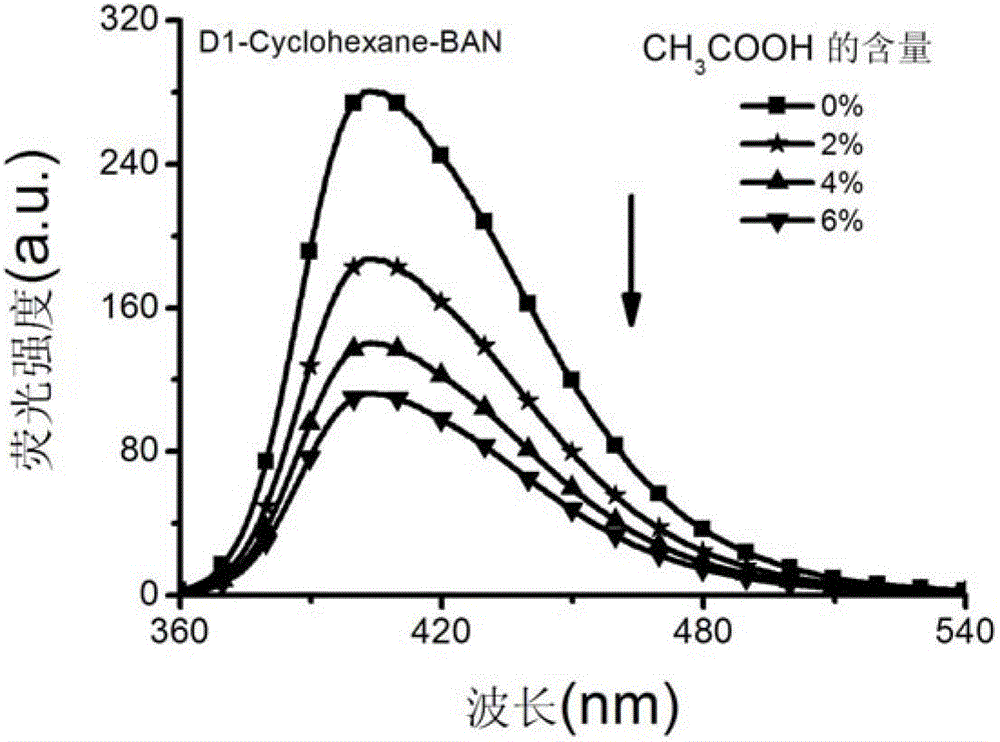
[0037] Preparation of 1-generation benzofuran naphthyridine (hereinafter referred to as: BAN) chromophore-modified dendrimer (D1-Cyclohexane-BAN) with 1,4-cyclohexanediamine as the core
[0038]
[0039] Add 3mmol of benzofurannaphthyridine (BAN) carboxylic acid derivative BAN-COOH and 15mL of DMSO into a 50mL two-neck flask, and then add 4.2mmol of 1-ethyl-(3-dimethylaminopropyl)carbonyl dicarboxylate after the solid is dissolved. Imine hydrochloride, 3 mmol 1-hydroxybenzotriazole (HOBT). Stir the reaction at room temperature under nitrogen protection for 2 hours, and slowly add 20 mL of 1-generation polyamide with cyclohexanediamine as the core in 20 mL dropwise to the reaction system - DMSO solution of amine dendrimer (G1-Cyclohexane), the ratio of the amount of BAN chromophore to the peripheral amine group of G1-Cyclohexane is 1:1, continue to stir and react for 96h, and slowly pour the reaction solution into 300mL water, filter and wash the solid with 30mL of water, an...
Embodiment 3
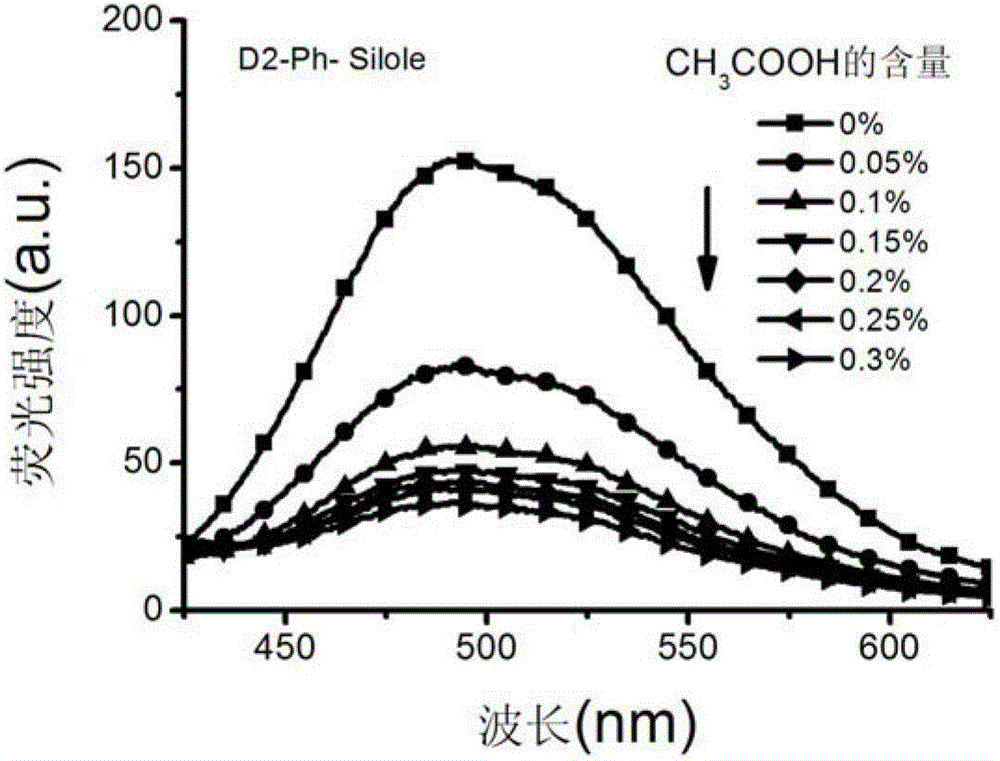
[0041] Preparation of 2-generation silacyclopentadiene chromophore-modified dendrimer (D2-Ph-Silole) with p-phenylenediamine as the core
[0042]
[0043] Add 3mmol of silole (Silole) carboxylic acid derivative Silole-COOH and 15mL of DMSO into a 50mL two-necked flask, and then add 4.2mmol of 1-ethyl-(3-dimethylaminopropyl)carbonyl Diimine hydrochloride, 3 mmol 1-hydroxybenzotriazole (HOBT). Stir the reaction at room temperature under nitrogen protection for 2 hours, slowly add 20 mL of 2-generation polyamide with 0.135 mmol p-phenylenediamine as the core to the reaction system dropwise - DMSO solution of amine dendrimer (G2-Ph), the ratio of Silole chromophore to G2-Ph peripheral amine group is 2:1, continue to stir and react for 96h, and slowly pour the reaction solution into 300mL water, filter and wash the solid with 30mL of water, and dry the crude product in a vacuum oven at 40°C. Dissolve the solid with 20 mL of chloroform, precipitate once in ether, filter and wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com