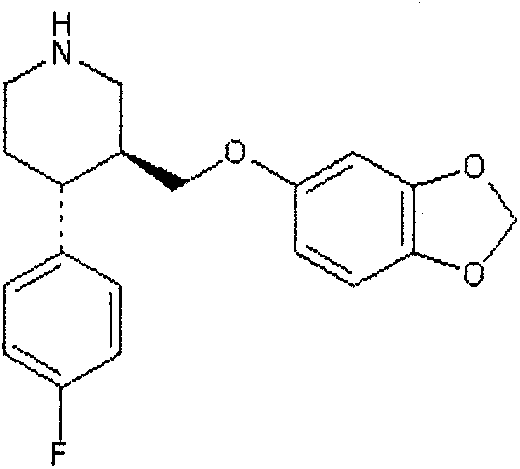
Paroxetine enteric-coated and sustained-release tablet and preparation method thereof
A technology for enteric and sustained-release tablets of paroxetine, which is applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve disadvantages, reduce production costs, drug prices, and difficulty in drug quality control. and risks, so as to enhance the recognition ability and improve the appearance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0044]
[0045]
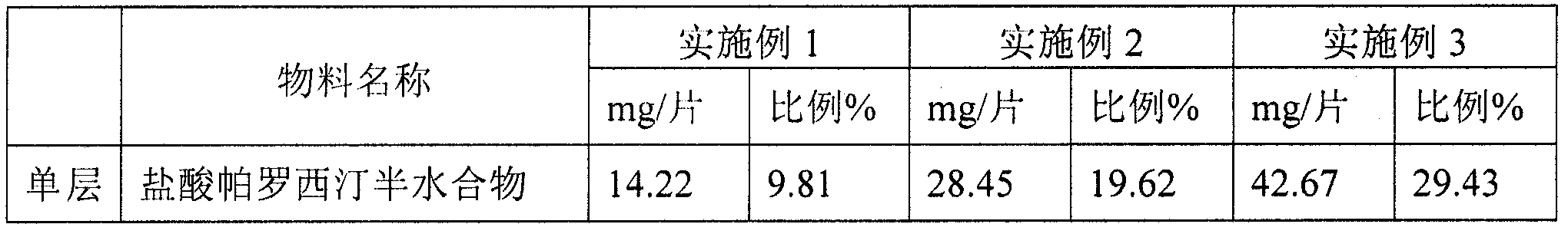
[0046] The particle size of paroxetine hydrochloride hemihydrate is D90=125 μm, D50=45 μm. 14.22mg of paroxetine hydrochloride hemihydrate is equivalent to 12.5mg of paroxetine, and so on for other specifications.
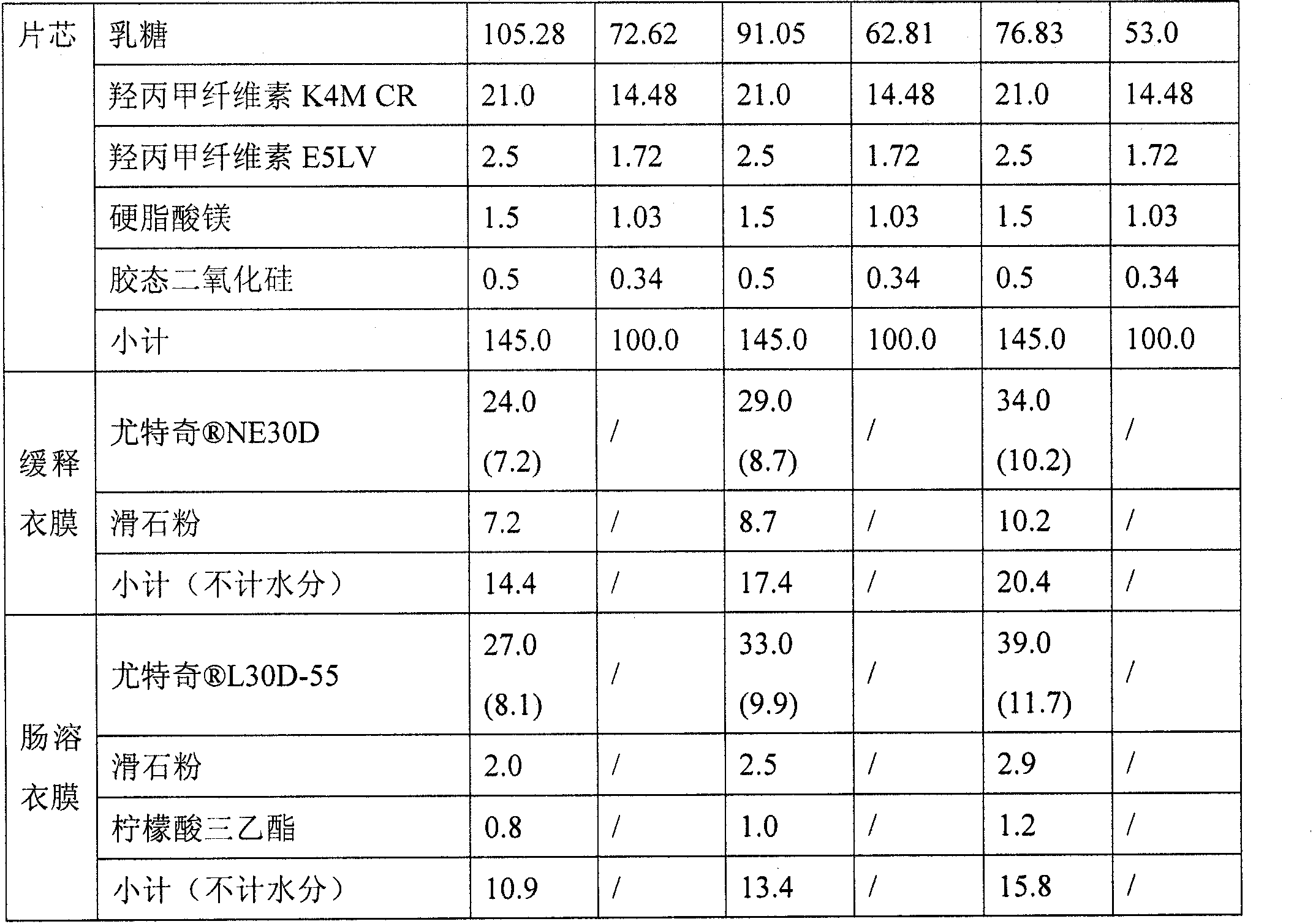
[0047] Both Eudragit NE30D and L30D-55 in the prescription are water dispersions with a solid content of 30%, and 24.0 (7.2) in the prescription indicates The dosage is 24.0mg, of which the solid content is 7.2mg.
[0048] Tablet core preparation method:
[0049] Active components, fillers, slow-release materials, and binders are mixed evenly, added with 5.8:1 (w / w) alcoholic water solution to granulate, dried in a fluidized bed, and colloidal silicon dioxide and stearin are added after granulation Magnesium acid mixed, compressed into tablets.
[0050] Extended Release Coating:
[0051] Disperse 100g of talc powder (D90=3.8μm) in 366.2g of purified water, stir well, then add 333.3g of After continuing to stir evenly, the above-mentio...
Embodiment 4-5
[0056]
Embodiment 6
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com