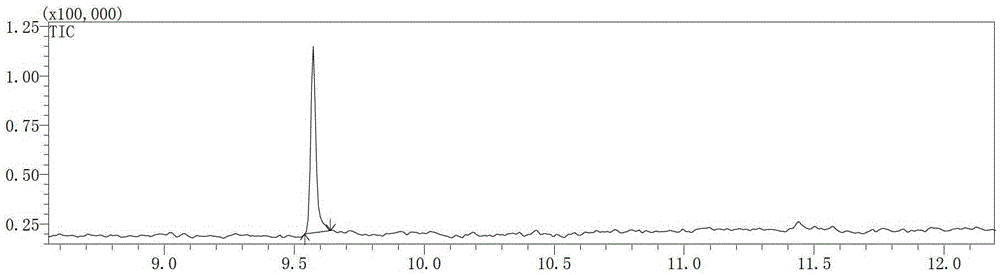
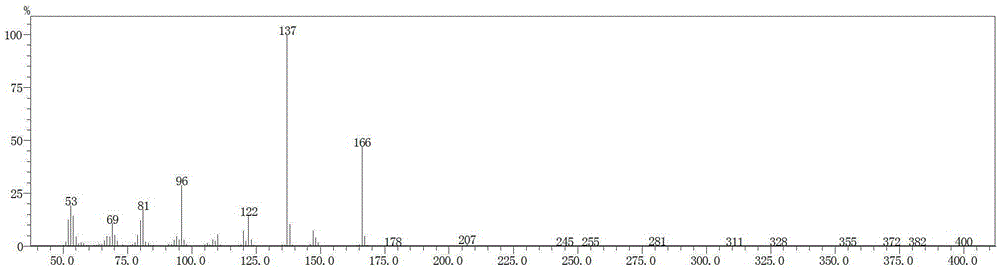
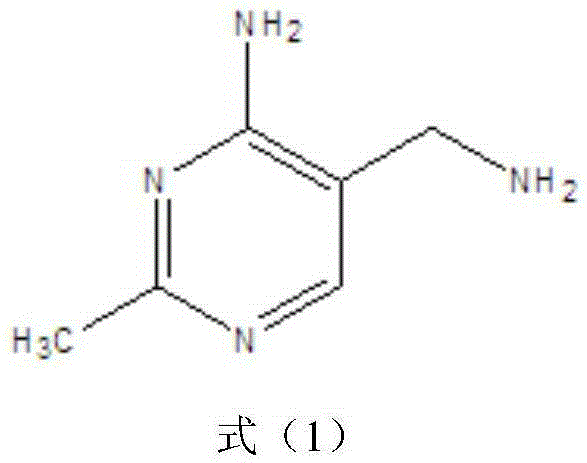
Environmentally friendly preparation method of vitamin B1 key intermediate 2-methyl-4-amino-5-aminomethylpyrimidine
A technology of aminomethylpyrimidine and amino group is applied in the production field of vitamin B1 and derivatives thereof, and can solve the problems of unfavorable environmental protection, difficulty in removing o-chloroaniline, and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1: With the functional polymer of 2-aminostyrene of formula (2-1) and sodium enolate as the starting reactant, the reaction formula is as follows:
[0104]
[0105] *: the compound of formula (4) reacts with acetamidine hydrochloride to generate 2-methyl-4-amino-5-formamidomethylpyrimidine (5), and simultaneously releases the functional polymer of aromatic amine structural unit (2-1 ).
[0106] Proceed as follows:
[0107] Add 200 g of water and 150 g of sodium enolate into a 500 ml four-neck flask, stir and dissolve at room temperature to obtain an aqueous solution of sodium enolate.
[0108] In another reactor, add 200 grams of water successively, 178.5 grams (1.5 moles) of formula (2-1) functional polymer (poly-2-aminostyrene) 150 grams of 30% dilute hydrochloric acid, 20 to 25 ℃ insulation reaction 30 minutes; then add the above-prepared aqueous solution of sodium enolate, heat the reaction at 20 to 30 ° C, and use the liquid phase to detect the conv...
Embodiment 2
[0111] The functional polymer (2-1) poly-2-aminostyrene that reclaims with embodiment 1 replaces new poly-2-aminostyrene, gets the polymkeric substance of 178.5 grams of 2-aminostyrene by dry weight, and enol Sodium reaction, the preparation steps and conditions are the same as in Example 1, and the purity and yield of the product obtained are shown in Table 1.
Embodiment 3
[0113] As described in Example 1, the difference is that the functional polymer is selected from formula (2-2) poly-4-aminostyrene, and the number average molecular weight range is 2000 to 12000; replace with 178.5 grams of polymer (2-2) The polymer (2-1) in Example 1 was reacted, and the preparation steps and conditions were the same as in Example 1. The purity and yield of the product obtained are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com