Method for reducing diacetylenic compounds
A compound and mixed solution technology, applied in the field of reducing diacetylenic compounds, can solve the problems of high price, large amount of tetrahydrofuran borane solution, large amount of borane, etc., and achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
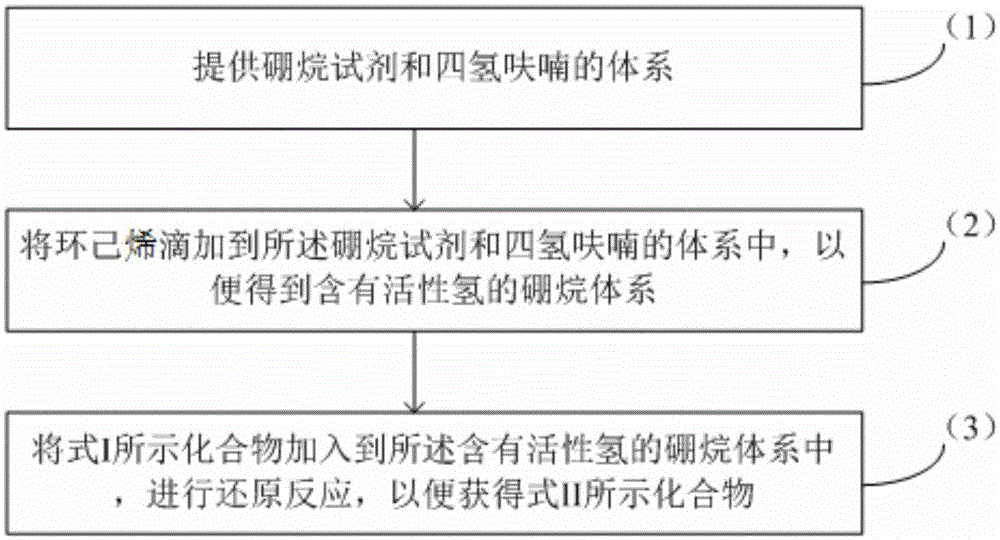
[0042] (1) Preparation of a system of borane reagent and tetrahydrofuran
[0043] Adding tetrahydrofuran into the reaction bottle, and then replacing the tetrahydrofuran with nitrogen, and then, under the protection of nitrogen, mixing the borane dimethyl sulfide solution and the nitrogen-substituted tetrahydrofuran to obtain a system of borane reagent and tetrahydrofuran.
[0044] (2) Synthesis of borane system containing active hydrogen
[0045] Cool the system of borane reagent and tetrahydrofuran obtained above to 0-10°C, then slowly add cyclohexene dropwise to the system of borane reagent and tetrahydrofuran, white solids will precipitate during the dropping process, and control The maximum temperature does not exceed 12°C. After the dropwise addition, the obtained mixture is kept at 0-10° C. for 3-5 hours to obtain a borane system containing active hydrogen, and this step does not need to be monitored.
[0046] (3) Synthesis of Diene Compounds
[0047] Cool the borane...
Embodiment 1
[0049] a. Synthesis of borane system containing active hydrogen
[0050] Add 150g (2.08mol) of tetrahydrofuran into a 500mL reaction flask, replace with nitrogen three times, add 35mL (0.35mol) of borane dimethyl sulfide solution under nitrogen protection, cool down to 0°C, and slowly add cyclohexene 63.3g (0.77 mol), the temperature was controlled not to exceed 12°C, and after the dropwise addition was completed, the reaction was incubated for 5 hours, and the reaction was stopped to obtain a borane system containing active hydrogen.
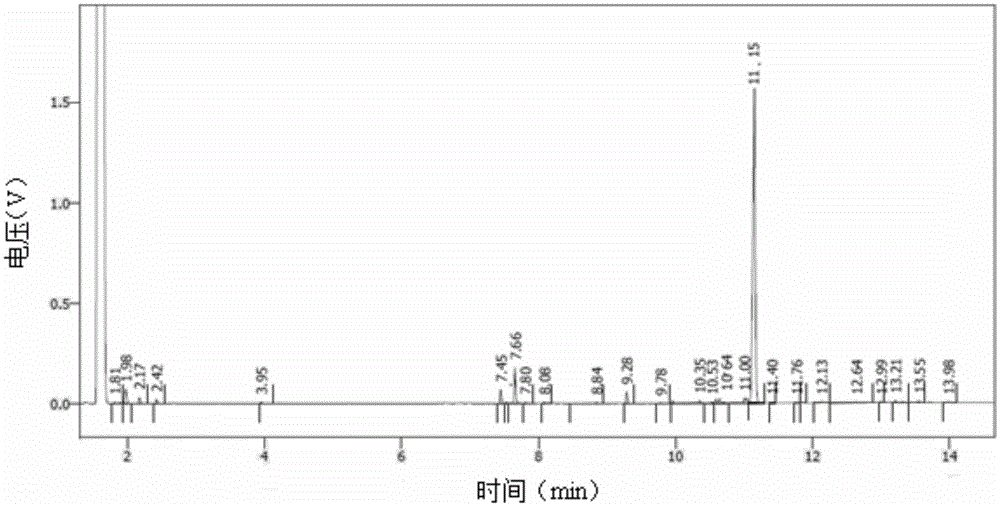
[0051] b. Reduction of 15,15-diethoxyhexadecyl-3,5-diyne
[0052] Cool the borane system containing active hydrogen obtained above to -10°C, slowly add 29.2 g (0.1 mol) of 15,15-diethoxyhexadecyl-3,5-diyne dropwise, after the dropwise addition is completed, Stir the reaction at -5-0°C for 5 hours, then raise the temperature to 5-10°C, stir the reaction for 4 hours, follow the reaction process by GC, control the raw material to be less than 1.5...
Embodiment 2
[0054] a. Synthesis of active hydrogen borane system
[0055] Add 150g (2.08mol) of tetrahydrofuran (THF) into a 500mL reaction flask, replace with nitrogen three times, add 40mL (0.4mol) of borane dimethyl sulfide solution under nitrogen protection, cool down to 10°C, and slowly add cyclohexene 69.8g (0.85 mol), control the temperature not to exceed 12°C, react for 4 hours after the dropwise addition, and stop the reaction to obtain a borane system containing active hydrogen.
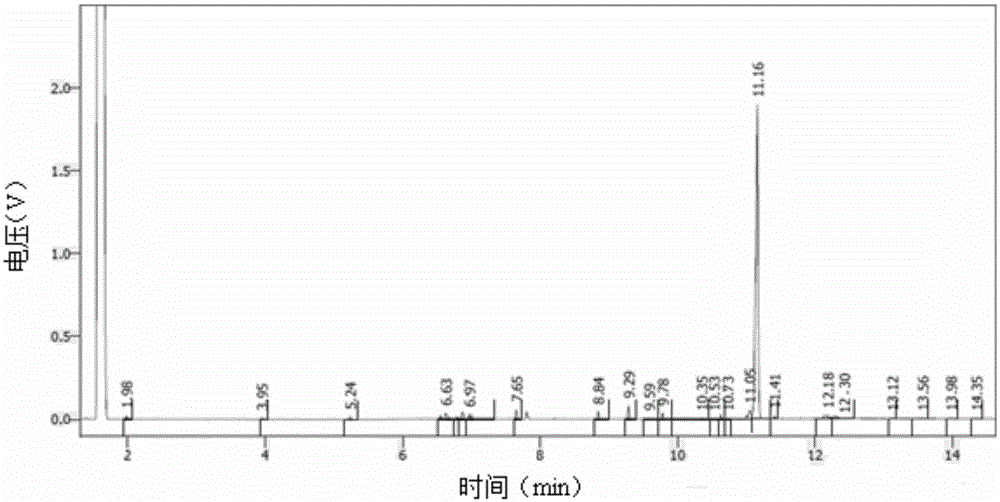
[0056] b. Reduction of 15,15-diethoxyhexadecyl-3,5-diyne
[0057] Cool the borane system containing active hydrogen obtained above to -10°C, slowly add 29.2 g (0.1 mol) of 15,15-diethoxyhexadecyl-3,5-diyne dropwise, after the dropwise addition is completed, Stir the reaction at -5-0°C for 5 hours, then raise the temperature to 5-10°C, stir the reaction for 4 hours, follow the reaction process by GC, control the raw material to be less than 1.5% (full integral), after the reaction is qualified, add 120...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com