Liquid formulations for ocular treatment
A technology of liquid preparation, rapamycin, applied in sensory diseases, allergic diseases, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0431] Example 1 - Preparation and Characterization of Rapamycin-Containing Solutions
[0432] 1.256% rapamycin (percentage of total weight) was dissolved in 9.676% ethanol (percentage of total weight). A 15% aqueous solution of F127 (Lutrol) in sterile water was slowly added with constant stirring. The final concentration was about 78.57% sterile water (percentage of total weight) and about 10.50% F127 (Lutrol) (percentage of total weight). This solution is listed in Table 1 as Formulation #32. The solution was kept at 2°C until use.
Embodiment 2
[0433] Example 2 - Subconjunctival injection of a solution containing rapamycin
[0434] 50 µl of the solution described in Example 1 was injected between the sclera and conjunctiva of New Zealand white rabbits.
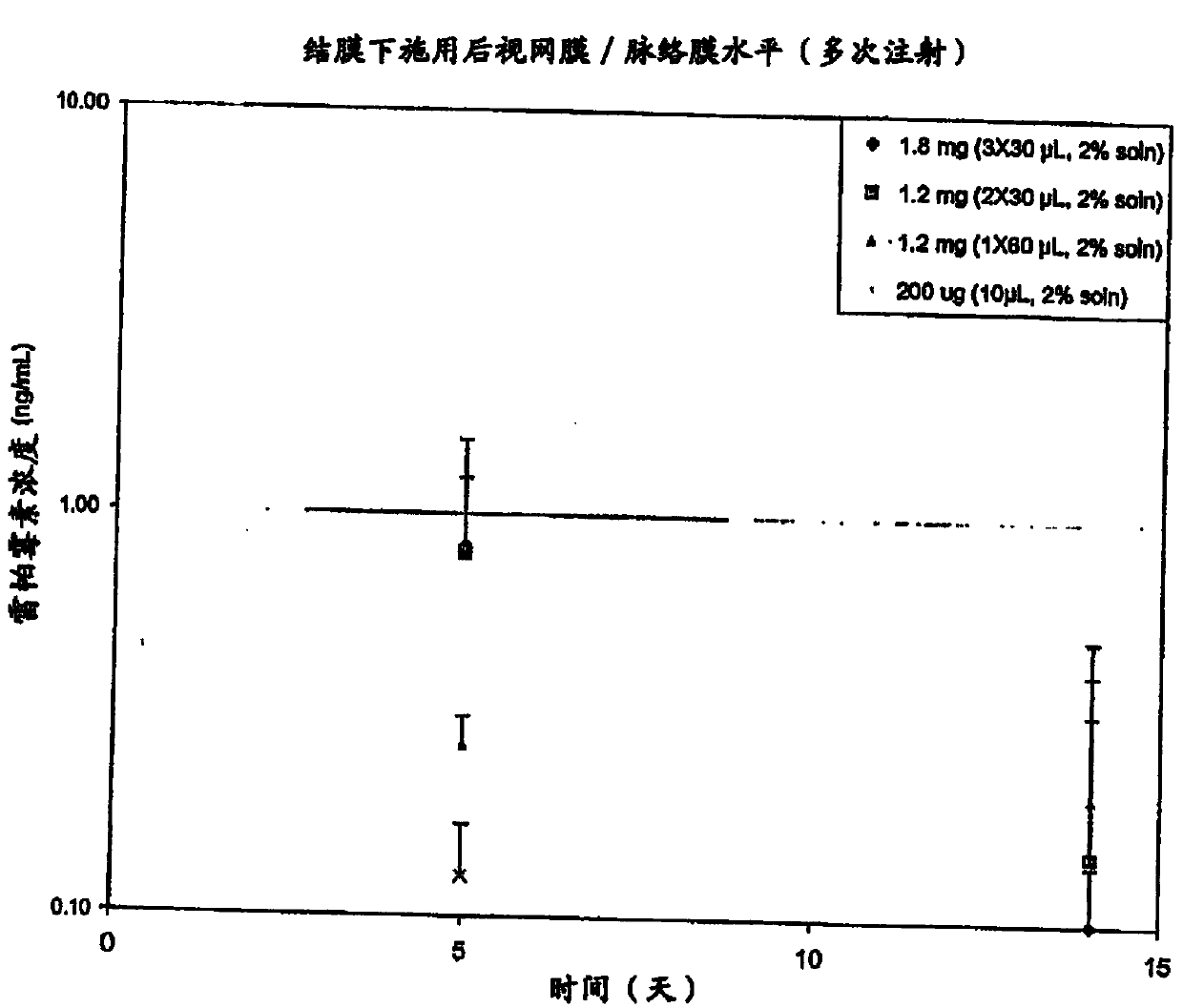
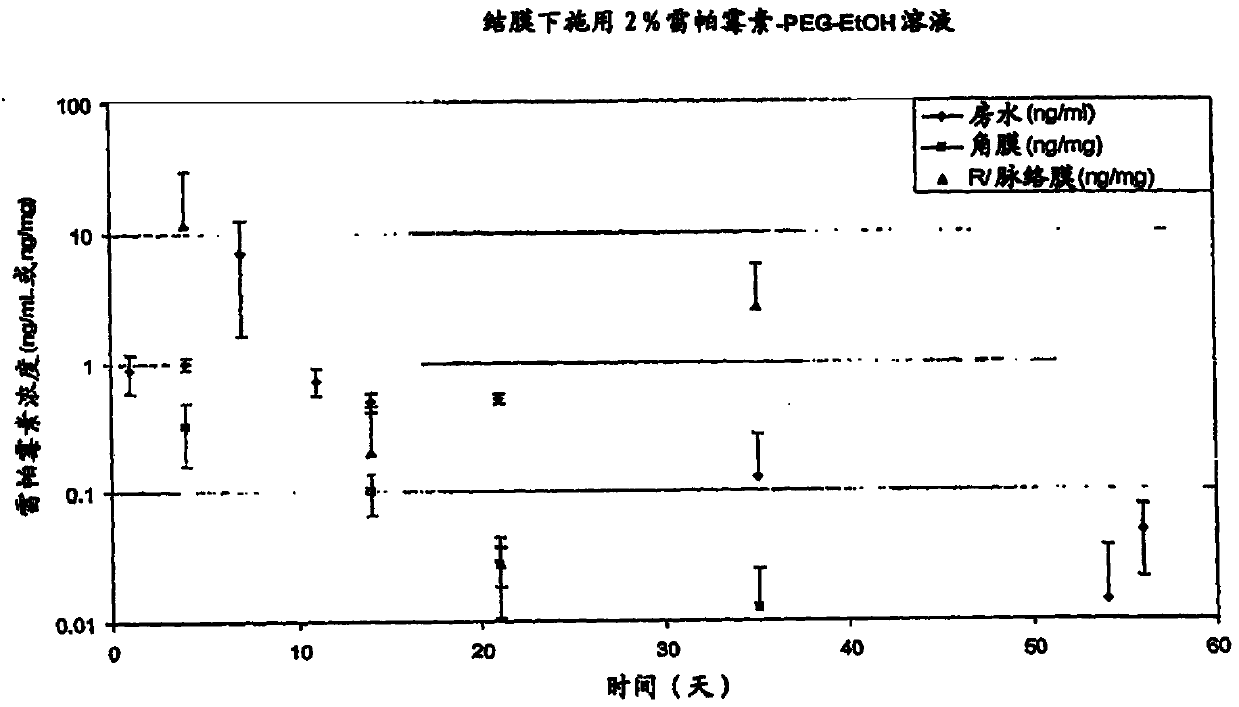
[0435] figure 2 The log scale mean concentrations of rapamycin present in the vitreous (ng / ml), retinachoroid (ng / mg) and sclera (ng / mg) 20, 40, 67 and 90 days after injection are depicted.
[0436] Analysis was performed by liquid chromatography mass spectrometry (LCMS) using an internal standard.
[0437] At each time point, the mean concentration of rapamycin was calculated by summing the obtained rapamycin concentrations per eye for each rabbit and dividing the number of eyes analyzed by the total amount. In this experiment, each time point represents the average of two individual eyes of two rabbits (four eyes at the time point) or the average of both eyes of one rabbit (two eyes at the time point).
[0438] Whole vitreous bodies were homogenized and analyze...
Embodiment 3
[0444] Example 3 - Preparation and Characterization of Rapamycin-Containing Solutions
[0445] 5.233% rapamycin (% of total formulation weight after addition of all ingredients) was dissolved in 0.4177g EtOH; the amount of EtOH was reduced to 0.1296g (6.344%, w / w) by forced evaporation (heating). Add PEG400 with constant stirring. The final concentrations as total weight percentages are about: rapamycin 5.233%, ethanol 6.344% and PEG4008 8.424%. On contact with the vitreous, the formulation forms non-dispersed masses relative to the surrounding medium. This solution is listed in Table 1 as Formulation #34.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com