Preparation method and application of a solid-supported catalyst for hydrocarbon oxidation
A technology of immobilized catalysts and hydrocarbons, which is applied in the direction of oxidation reaction preparation, organic compound preparation, physical/chemical process catalysts, etc. It can solve the problem that catalysts are difficult to separate and recycle, and achieve many steps in the immobilization process and simple preparation methods , the complex effect of the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
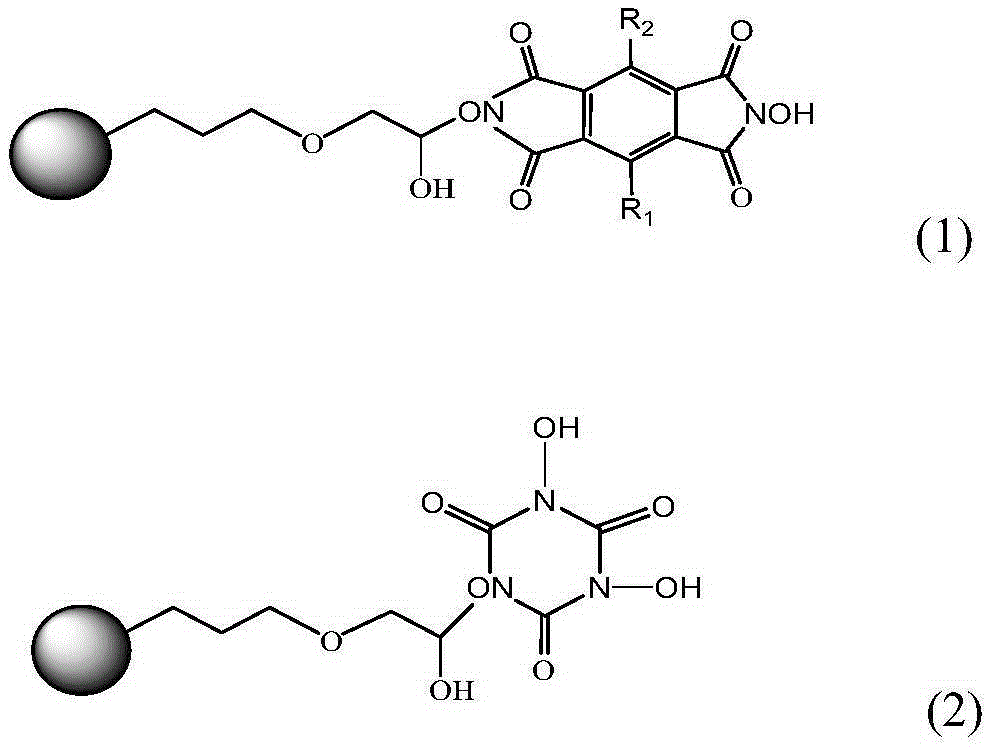
[0031] The immobilized N,N'-dihydroxypyrylenetetracarboximide catalyst was prepared according to the following procedure.
[0032]
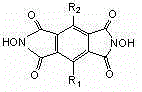
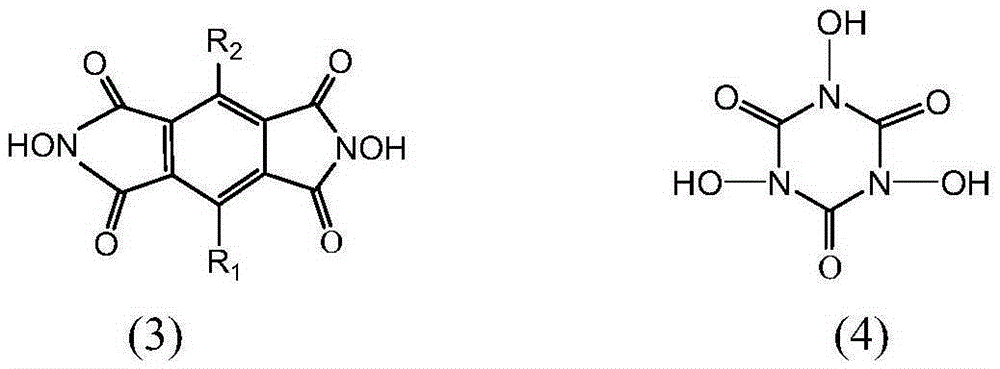
[0033] 1.34g (19.2mmol) of hydroxylamine hydrochloride and 2.6mL (19mmol) of triethylamine were dissolved in 60mL of ethanol, stirred for 10min, and 2.18g (9.8mmol) of 1,2,4,5-benzenetetracarboxylic anhydride [formula (5 )], stirred and refluxed under nitrogen atmosphere for 8h, cooled to room temperature, added 100ml deionized water, stirred evenly and precipitated a yellow solid. The solid filtered out with suction was washed several times with deionized water, and dried under vacuum to obtain N,N'-dihydroxypyrolitetetridine (NDHPI) [formula (6)].
[0034] After activation treatment, 4.00g of commercially available SBA-15 (Nanjing Jicang Nano Technology Co., Ltd.) was suspended in 120ml of toluene, and 0.012mol (2.83g) of γ-(2,3 glycidyl oxide) was added while fully stirring. Base) propyltrimethoxysilane, 110 ° C nitrogen protection reflux ...
Embodiment 2
[0037] In a 100ml passivated autoclave, add 1.37g of immobilized N,N'-dihydroxypyrylenetetracarboximine prepared in Example 1, 0.0036g of N,N-disalicylaldehyde ethylenediamine cobalt complex (Cosalen) , 4.00g of acetonitrile, 5.00g of toluene, 1.60MPa oxygen gas was introduced to react at one time, the reaction temperature was 90°C, after 7h, the reaction kettle was taken out and cooled rapidly, and the reaction solution was taken out after pressure relief, analyzed by gas chromatography, and cyclohexanone was used as The internal standard was used for quantification, and the conversion rate of toluene was measured to be 12.74%, and the selectivities of benzaldehyde, benzyl alcohol and benzoic acid were 40.31%, 9.25% and 50.43%, respectively.
Embodiment 3
[0039] In a 100ml passivated autoclave, add 1.37g of immobilized N,N'-dihydroxypyrylenetetracarboximine prepared in Example 1, 0.0036g of N,N-disalicylaldehyde ethylenediamine cobalt complex (Cosalen) , 4.00g of acetonitrile, 5.00g of toluene, 1.60MPa oxygen was introduced into the reaction at one time, the reaction temperature was 110°C, after 4h, the reaction kettle was taken out and cooled rapidly, and the reaction solution was taken out after pressure relief, analyzed by gas chromatography, and cyclohexanone was used as The internal standard was used for quantification, and the conversion rate of toluene was measured to be 52.25%, and the selectivities of benzaldehyde, benzyl alcohol and benzoic acid were 17.81%, 5.16% and 77.02%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com