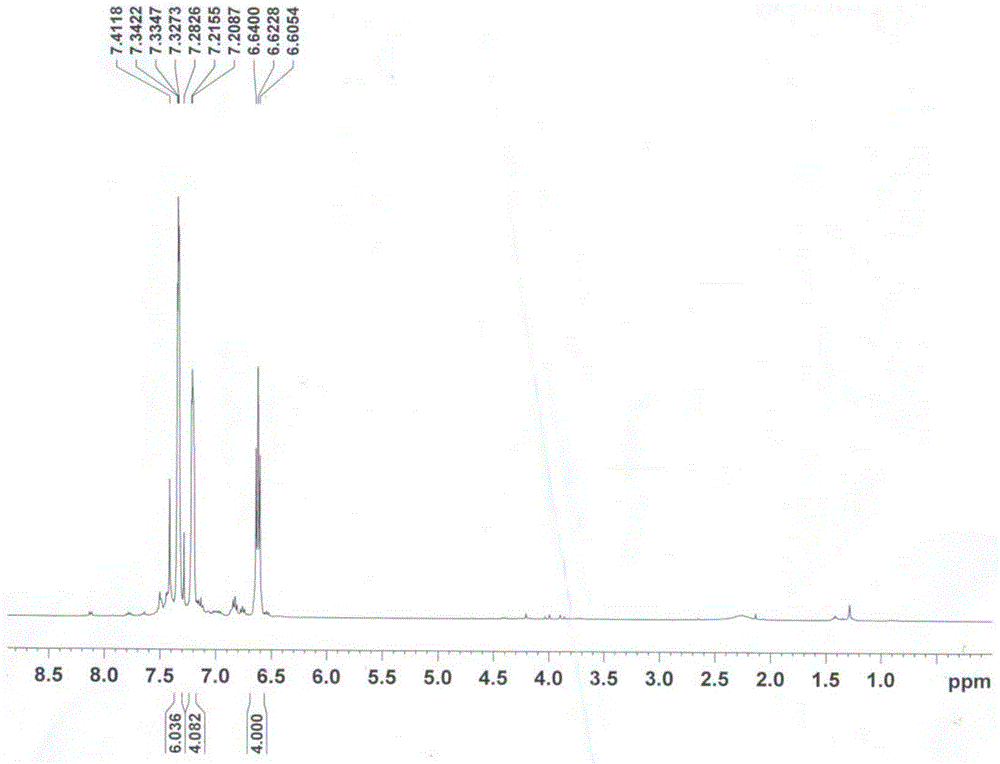
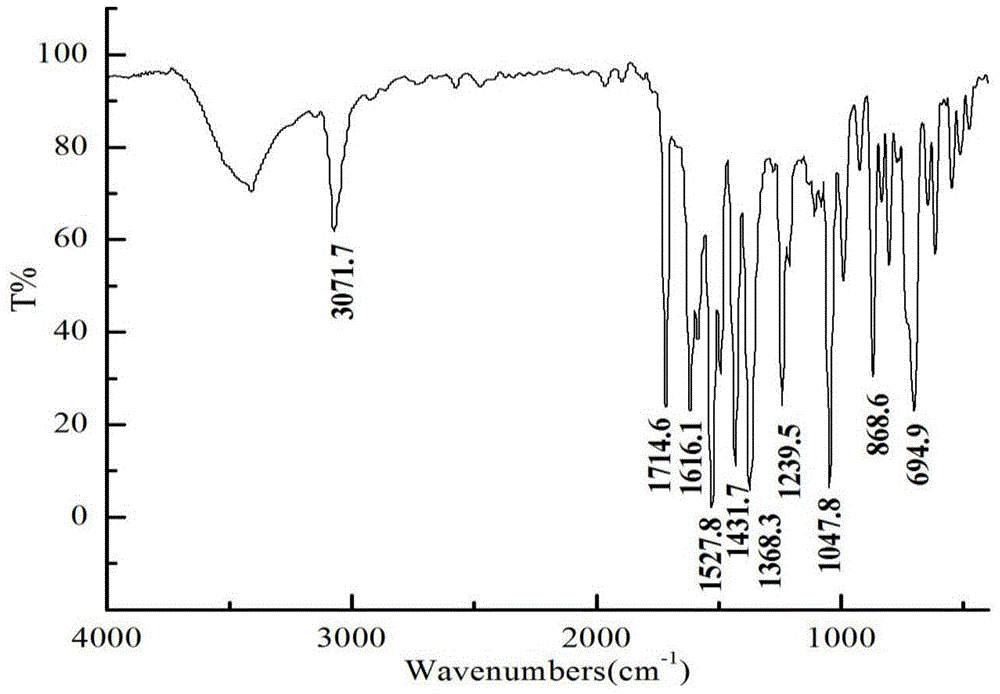
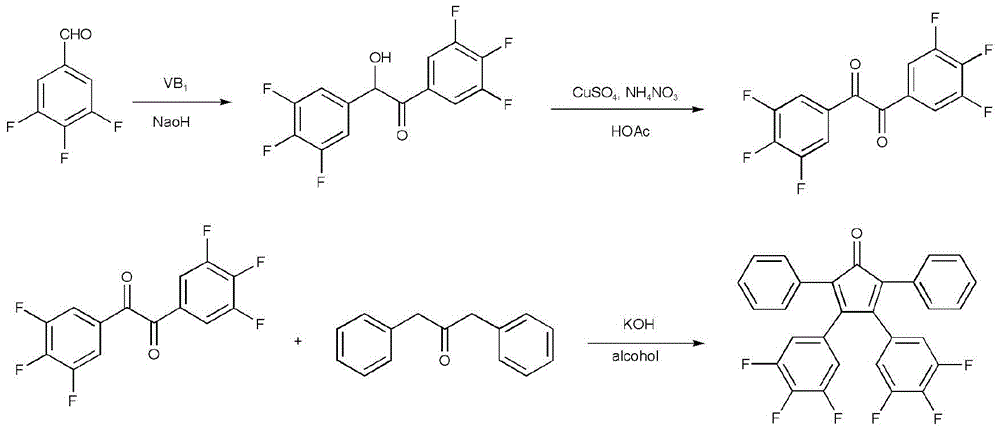
A kind of preparation method of 3,4-bis(3,4,5-trifluorophenyl)-2,5-diphenylcyclopentadienone
A technology of diphenylcyclopentadienone and trifluorophenyl, which is applied in the field of organic compound synthesis, can solve the problems of increasing the difficulty of steric hindrance synthesis reaction, unfavorable industrial production, and no literature reports, etc. Safe and reliable, easy to separate and purify, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethanone
[0034] Under ice-water bath, add 1.8gVB successively to the reactor 1 , 5.0mL of distilled water, 15.0mL of absolute ethanol, and keep it for 10min, then add 11.0mL of 3,4,5-trifluorobenzaldehyde, slowly add dropwise 3.0mol / L sodium hydroxide ethanol solution at 3°C to the solution with stirring The pH value was 9. After reacting for 45 minutes, it was heated to 60°C and reacted for another 90 minutes. Cool to room temperature, filter with suction, wash the filter cake three times with distilled water, and the filtrate is neutral, then recrystallize with ethanol to obtain 10.3g of 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethyl Ketone white crystals, m.p: 76-78°C (literature value: 75-77°C), yield 68.0%.
[0035] (2) Preparation of 3,4,5-ditrifluorobenzil
[0036]Add 10.3g 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethanone, 10.0g ammonium nitrate, 0.4g anhydrous copper sulfate, 25.0mL glacial acetic ...
Embodiment 2
[0044] (1) Preparation of 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethanone
[0045] Under the ice-water bath, add 3.6gVB successively to the reactor 1 , 10.0mL distilled water, 30.0mL absolute ethanol, and keep it for 20min, then add 22.0mL 3,4,5-trifluorobenzaldehyde, slowly add 3.0mol / L sodium hydroxide ethanol solution at 0°C dropwise under stirring to the solution The pH value was 9. After reacting for 45 minutes, it was heated to 70°C and reacted for another 2 hours. Cool to room temperature, filter with suction, wash the filter cake three times with distilled water, and the filtrate is neutral, then recrystallize with ethanol to obtain 21.8 g of 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethyl Ketone white crystals, m.p: 74-76°C, yield 71.8%.
[0046] (2) Preparation of 3,4,5-ditrifluorobenzil
[0047] Add 21.8g 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethanone, 20.0g ammonium nitrate, 0.8g anhydrous copper sulfate, 50.0mL glacial acetic acid, 20.0mL water, stirred and w...
Embodiment 3
[0051] (1) Preparation of 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethanone
[0052] Under ice-water bath, add 1.8gVB successively to the reactor 1 , 5.0mL distilled water, 15.0mL absolute ethanol, and keep it for 10min, then add 11.0mL 3,4,5-trifluorobenzaldehyde, slowly add 3.0mol / L sodium hydroxide ethanol solution at 5°C dropwise under stirring to the pH of the solution The value is 10. After reacting for 50 minutes, heat to 65° C. and react for another 3 hours. Cool to room temperature, filter with suction, wash the filter cake three times with distilled water, and the filtrate is neutral, then recrystallize with ethanol to obtain 10.6g of 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethyl Ketone white crystals, m.p: 76-79°C, yield 70.2%.
[0053] (2) Preparation of 3,4,5-ditrifluorobenzil
[0054] Add 10.6g 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethanone, 10.0g ammonium nitrate, 0.4g anhydrous copper sulfate, 25.0mL glacial acetic acid, 10.0mL water, stirred and heated to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com