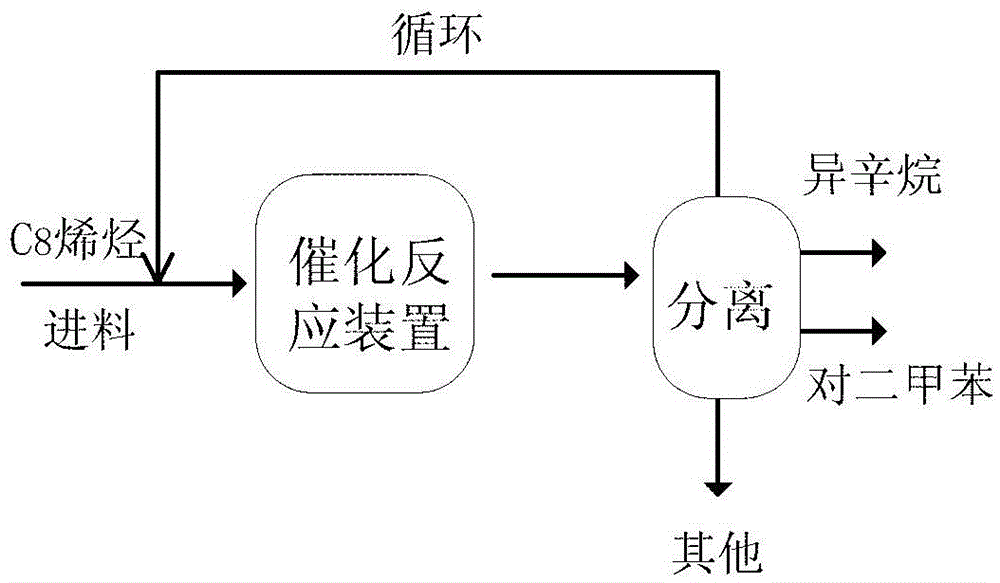
A method for preparing p-xylene and co-producing isooctane from diisobutylene
A diisobutene and p-xylene technology, applied in the direction of isomerization hydrocarbon production, chemical instruments and methods, hydrogenation hydrocarbon production, etc., can solve the problems not involving p-xylene and isooctane processes, etc., and achieve improved aromatization efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0030] Embodiment 1~6 prepares the catalyst used
Embodiment 1
[0031] Embodiment 1 (Cr 2 o 3 ) 0.05 (MgO) 0.01 PD 0.02 (Al 2 o 3 ) 0.92 preparation of
[0032] Prepare the catalyst by impregnation method according to the above metering ratio, dissolve 26.3g of chromium nitrate nonahydrate and 6.4g of magnesium nitrate hexahydrate in 500mL of deionized water, and then add 92g of γ-Al to the solution 2 o 3 The carrier was impregnated with stirring for 24 hours, evaporated to dryness, and then calcined at 600°C in the air for 4 hours; 5.0 g of palladium nitrate was dissolved in 500 mL of deionized water to prepare a solution, and the calcined solid was immersed in the palladium nitrate solution under stirring conditions for 24 hours. Evaporated to dryness, and then calcined in air at 400°C for 4h. At 450°C, pass through hydrogen-nitrogen mixed gas for reduction to obtain the above-mentioned catalyst.
Embodiment 2
[0033] Embodiment 2 (Cr 2 o 3 ) 0.10 (K 2 O) 0.015 Ir 0.01 (Al 2 o 3 ) 0.875 preparation of
[0034] Prepare the catalyst by the sol-gel method according to the above metering ratio, dissolve 350.5g of aluminum isopropoxide in 500mL of absolute ethanol, dissolve 52.7g of chromium nitrate and 3.2g of potassium nitrate in 200mL of deionized water, and drop into isopropoxide under rapid stirring. Aluminum propoxide ethanol solution, adjust the pH to 7 with ammonia water, make the solution into a gel, and become a gel after standing for 24 hours; then calcined in the air at 500°C for 5 hours; take 2.3g of ammonium chloroiridate dissolved in 500mL of deionized water to prepare Solution, the calcined solid is immersed in the ammonium chloroiridate solution under stirring conditions, soaked for 24 hours, dried and calcined in the air at 400°C for 4h, after the calcining is completed, the hydrogen-nitrogen mixture gas is introduced at 450°C for reduction, and the above-mention...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com