common light chain mouse
A mouse, light chain technology, applied in the field of genetically modified mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Identification of the human heavy chain variable region combined with the selected human light chain variable region
[0193] An in vitro expression system was constructed to determine whether a single rearranged human germline light chain can be co-expressed with a human heavy chain derived from an antigen-specific human antibody.
[0194] Methods of producing human antibodies in genetically modified mice are known (see, for example, US 6,596,541, Regeneron Pharmaceuticals, ). The technology involves generating genetically modified mice having a genome that includes human heavy and light chain variable regions operably linked to endogenous mouse constant region loci, so that the mouse responds to antigen stimulation to produce human variable regions. And mouse constant region antibodies. Coded by The DNA of the variable regions of the heavy and light chains of antibodies produced in mice is fully human. Initially, high-affinity chimeric antibodies were isolated with hum...
Embodiment 2
[0206] Generation of rearranged human germline light chain loci
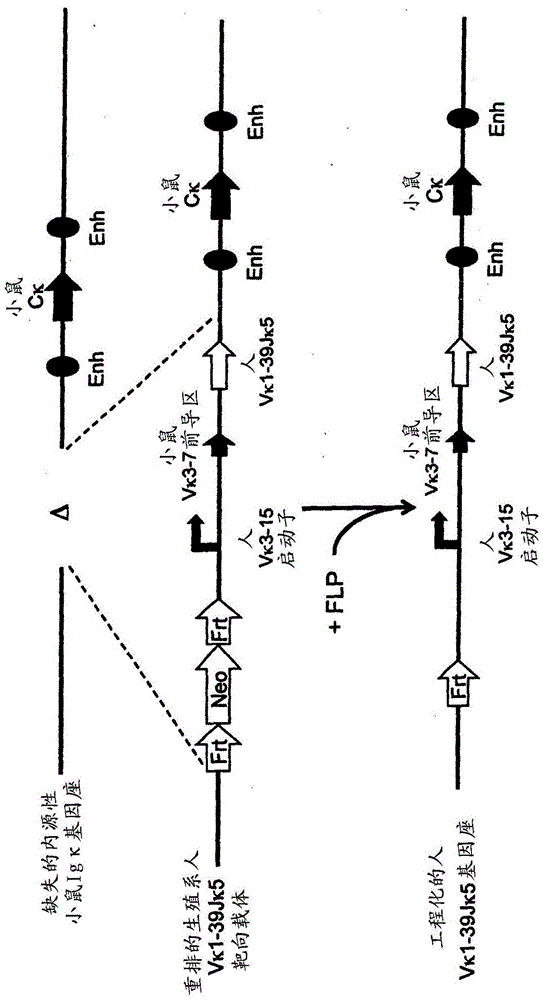
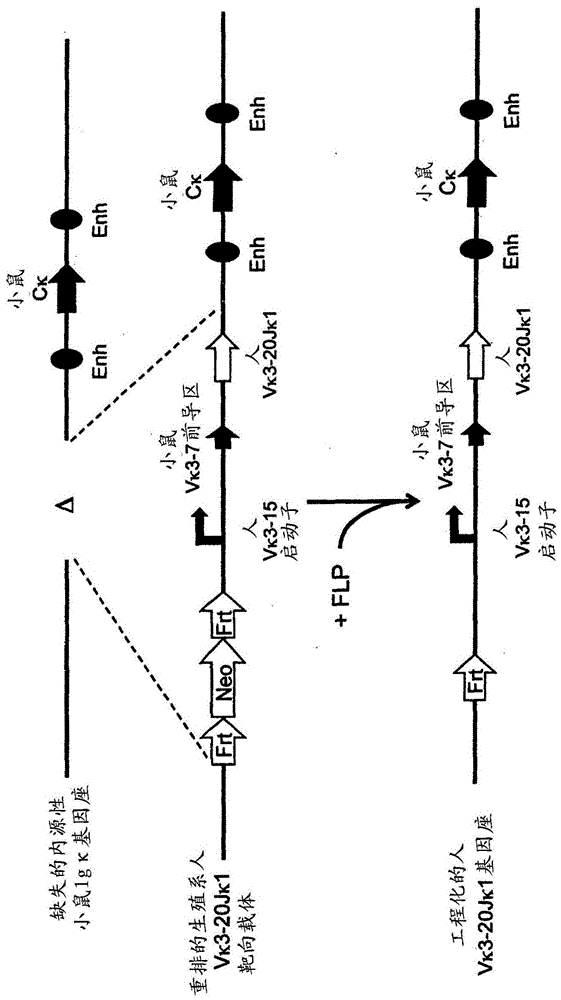
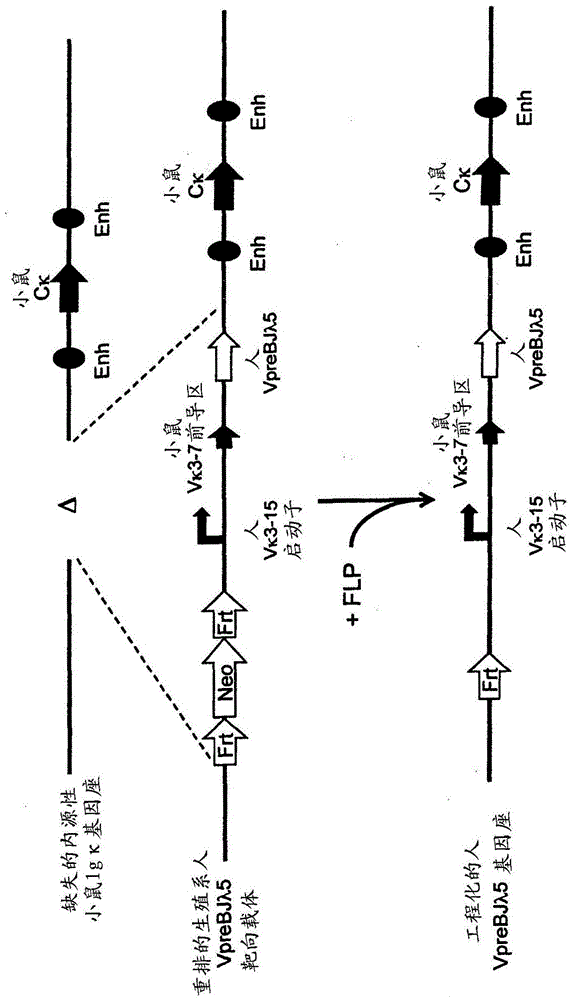
[0207] use Technology to prepare various rearranged human germline light chain targeting vectors (see, for example, US Pat. No. 6,586,251 and Valenzuela et al. (2003) High-throughput engineering of the mouse genome coupled with high-resolution expression analysis, Nature Biotech. 21(6):652-659) to modify the mouse genome bacterial artificial chromosome (Bacterial Artificial Chromosome, BAC) clones 302g12 and 254m04 (Invitrogen). Using these two BAC clones, the genomic construct was engineered to protect a single rearranged human germline light chain region and inserted into an endogenous kappa light chain locus, which was previously modified to delete the endogenous kappa Change and connect gene fragments.
[0208] Construction of a rearranged human germline light chain targeting vector. Three different rearranged human germline light chain regions were prepared using known standard molecular biology techniques. ...
Embodiment 3
[0224] Generation of mice expressing a single rearranged human light chain
[0225] The above target ES cells were used as donor ES cells and passed Method to introduce 8-cell stage mouse embryos (see, for example, US Pat. No. 7,294,754 and Poueymirou et al. (2007) F0 generationmice that are essentially fully derived from the donor gene-targeted ES cells allowing immediate phenotypic analyses Nature Biotech. 25(1) :91-99.). Carrying the engineered human germline Vκ1-39Jκ5 light chain region, Vκ3-20Jκ1 light chain region or VpreBJλ5 light chain region independently Allelic modification analysis was used to detect the presence of uniquely rearranged human germline light chain regions through genotyping (Valenzuela et al., supra).
[0226] The pups were genotyped and the pups heterozygous or homozygous for the rearranged human germline light chain region were selected to characterize the expression of the rearranged human germline light chain region.
[0227] Flow Cytometry. The exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com